甲氧滴滴涕 | 72-43-5
中文名称
甲氧滴滴涕
中文别名
1,1,1-三氯-2,2-双对甲氧苯基乙烷;甲氧DDT;甲氧氯;甲氧滴滴涕,甲氧氯;2,2-二对茴香基-1,1,1-三氯乙烷;2-2-双(对-甲氧苯基)-1,1,1-三氧乙烷;灭虫氧氯
英文名称
Methoxychlor
英文别名
1-methoxy-4-[2,2,2-trichloro-1-(4-methoxyphenyl)ethyl]benzene
CAS
72-43-5
化学式
C16H15Cl3O2
mdl
MFCD00000803
分子量
345.653
InChiKey
IAKOZHOLGAGEJT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:86-88 °C(lit.)
-
沸点:346 °C
-
密度:1.41 g/cm3 (25℃)
-
闪点:11 °C
-
溶解度:Soluble in ethanol (Windholz et al., 1983), chloroform (440 g/kg), xylene (440 g/kg), and methanol (50 g/kg) (Worthing and Hance, 1991)
-
暴露限值:NIOSH REL: IDLH 5,000 mg/m3; OSHA PEL: TWA 15 mg/m3; ACGIH TLV: TWA 10 mg/m3.
-
物理描述:Methoxychlor is a white crystalline solid which is often dissolved in a liquid carrier such as diesel oil. It can cause illness by inhalation, skin absorption and/or ingestion. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. If dissolved in a liquid carrier, it can easily penetrate the soil and contaminate groundwater and nearby streams. It is used as a pesticide.
-
颜色/状态:Colorless crystals (technical, grey powder)
-
气味:Slight, fruity odor
-
蒸汽密度:12 (NTP, 1992) (Relative to Air)
-
蒸汽压力:4.2X10-5 mm Hg at 25 °C (est)
-
亨利常数:2.03e-07 atm-m3/mole
-
稳定性/保质期:
对兔皮肤和眼睛有轻度刺激作用。
-
分解:... When heated to decomp, it emits highly toxic fumes of /hydrogen chloride/ .
-
腐蚀性:Methoxychlor will attack some forms of plastics, rubbers, and coatings.
-
气味阈值:4.7 mg/kg in water
-
保留指数:2439.3;2413;2410;2401;2418;2426.5;2416.9;2395;2450.6;2417.7;2417;2420;2436.7;2420;2420;2417;2399.4
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:21
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
尽管甲氧滴滴涕(methoxychlor)通过类似于滴滴涕(DDT)的途径以较小的程度被缓慢代谢,其主要代谢途径是通过邻位去甲基化(o-demethylation)和随后的结合反应。这些途径在哺乳动物的微粒体酶和土壤生物的酶的催化下进行。
Although methoxychlor is slowly metabolized to small extent by pathways similar to those of DDT. ... the major ... pathway ... is by o-demethylation and subsequent conjugation. ... These pathways are catalyzed by microsomal enzymes in mammals and by enzymes in soil organisms ... .
来源:Hazardous Substances Data Bank (HSDB)
代谢
在老鼠中,口服标记的甲氧氯在24小时内消除了98%。主要的代谢途径是o-脱烷基化,生成2-(对-羟基苯基)-2-(对-甲氧基苯基)-1,1,1-三氯乙烷,2,2-双(对-羟基苯基)-1,1,1-三氯乙烷及其乙烯,以及4,4'-二羟基苯甲酮,这些代谢物主要以结合物的形式被排出体外。
In mice, 98% of labeled methoxychlor given orally was eliminated within 24 hr. Main metabolic pathway ... o-dealkylation to 2-(para-hydroxyphenyl)-2-(para-methoxyphenyl)-1,1,1-trichloroethane, 2,2-bis(para-hydroxyphenyl)-1,1,1-trichloroethane and its ethylene, and 4,4'-dihydroxybenzophenone which were excreted mainly as conjugates.
来源:Hazardous Substances Data Bank (HSDB)
代谢
(14)C标记的甲氧氯通过口服给予完整、结肠造口和胆汁瘘的鸡。在相似的剂量和时间下,母鸡尸体中的(14)C回收率高于公鸡。蛋中的(14)C随剂量和时间变化,并且主要存在于蛋黄中。确定了或表征了26种代谢物:粪便中22种,尿液中14种,胆汁中6种。代谢包括去甲基化、环羟基化、与葡萄糖醛酸结合、脱氯、脱氢氯化以及取代苯并苯酮的形成。文章中提供了一些代谢物的化学结构。
(14)C-labeled methoxychlor was given orally to intact, colostomized, and bile-fistulated chickens. Recovery of (14)C was greater in carcasses of hens compared to roosters for comparable dosages and time. (14)C in eggs varied with dose and time and was predominantly in yolk. 26 metabolites were either identified or characterized: 22 metabolites in feces, 14 in urine, and 6 in bile. Metabolism incl demethylation, ring hydroxylation, conjugation with glucuronic acid, dechlorination, dehydrochlorination, and formation of substituted benzophenones. Chem structures of some metabolites are provided in the article.
来源:Hazardous Substances Data Bank (HSDB)
代谢
(14)C标记的甲氧氯通过口服给予一个胆管插管的山羊,并通过皮肤表面给予另外两只山羊。在3天结束时,从胆汁中分离出7种代谢物以及甲氧氯。这些包括1,1-二氯-2,2-双(4-甲氧基苯基)乙烯、1,1-二氯-2,2-双(4-甲氧基苯基)乙烷、1,1,1-三氯-2-(4-羟基苯基)-2-(4-甲氧基苯基)乙烷、1,1-二氯-2-(4-羟基苯基)-2-(4-甲氧基苯基)乙烯、1,1-二氯-2-(4-羟基苯基)-2-(4-甲氧基苯基)乙烷、1,1-二氯-2,2-双(4-羟基苯基)乙烯和1,1-二氯-2,2-双-(4-羟基苯基)乙烷。
(14)C-labeled methoxychlor was given orally to a bile-cannulated goat and via skin surface to 2 other goats. At the end of 3 days, seven metabolites plus methoxychlor were isolated from the bile. These were 1,1-dichloro-2,2-bis(4-methoxyphenyl)ethene, 1,1-dichloro-2,2-bis(4-methoxyphenyl)ethane, 1,1,1-trichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethane, 1,1-dichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethene, 1,1-dichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethane, 1,1-dichloro-2,2-bis(4-hydroxyphenyl)ethene, and 1,1-dichloro-2,2-bis-(4-hydroxyphenyl)ethane.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Methoxychlor has known human metabolites that include Mono-hydroxy-methoxychlor.
来源:NORMAN Suspect List Exchange
毒理性
甲氧氯的某些单羟基和双羟基代谢物,特别是2,2-双(对-羟基苯基)-1,1,1-三氯乙烷(HPTE),作为雌激素类似物发挥作用。HPTE是雌激素受体α的激动剂,同时也在雌激素受体β和雄激素受体上发挥拮抗作用。这影响了蛋白质的合成,据信这是甲氧氯许多雌激素效应的原因,如降低生育能力。由于甲氧氯也是DDT的结构类似物,因此它被认为具有相同的神经毒性效应。这包括阻止神经元激活和膜去极化后钠通道的失活,导致神经的超兴奋性。与DDT一样,甲氧氯也可能抑制神经元的腺苷三磷酸酶(ATP酶),尤其是Na+K+-ATP酶和Ca2+-ATP酶,它们在神经元复极化中起着至关重要的作用。这导致去极化速率降低,并增加了神经元对小型刺激的敏感性,这些刺激在完全去极化的神经元中不会引起反应。(T10,A118)
Certain mono- and bis-hydroxy metabolites of methoxychlor, especially 2,2-bis(p-hydroxyphenyl)-1,1, 1-trichloroethane (HPTE), act as estrogen analogues. HPTE is an estrogen receptor alpha agonist, and also acts as an antagonist at the estrogen receptor beta and androgen receptor. This affects protein synthesis, which is believed to cause many of methoxychlor's estrogenic effects, such as decreased fertility. As methoxychlor is also a structural analogue of DDT, it is believed to have the same neurotoxic effects. This includes preventing the deactivation of the sodium gate after neuron activation and membrane depolarization, resulting in hyperexcitability of the nerve. Like DDT, methoxychlor may also inhibit neuronal adenosine triphosphatases (ATPases), particularly Na+K+-ATPase and Ca2+-ATPase, which play vital roles in neuronal repolarization. This contributes to the reduced rate of depolarization and increases the sensitivity of neurons to small stimuli that would not elicit a response in a fully depolarized neuron. (T10, A118)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
致癌性分类:1)人类证据:无数据;2)动物证据:不足。对人类致癌风险的总体评估为第3组:该物质对人类致癌性不可分类。/来自表格/
Classification of carcinogenicity: 1) evidence in humans: no data; 2) evidence in animals: insufficient. Overall summary evaluation of carcinogenic risk to humans is Group 3: The agent is not classifiable as to its carcinogenicity to humans. /From table/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
癌症分类:D组 不可归类为人类致癌性
Cancer Classification: Group D Not Classifiable as to Human Carcinogenicity
来源:Hazardous Substances Data Bank (HSDB)
毒理性
分类:D;无法归类为人类致癌性。分类依据:人类数据不可用,动物证据不确定。人类致癌性数据:无。/基于先前的分类系统/
CLASSIFICATION: D; not classifiable as to human carcinogenicity. BASIS FOR CLASSIFICATION: Human data are unavailable, and animal evidence is inconclusive. HUMAN CARCINOGENICITY DATA: None. /Based on a former classification system/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A4:不能分类为人类致癌物。
A4: Not classifiable as a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
When dissolved in oil or other lipid, they are all readily absorbed by the skin and alimentary canal. /All chlorinated hydrocarbon insecticides/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
喂食标记甲氧滴滴涕的老鼠收集了尿液和粪便。在回收的放射性物质中,90%在粪便中,10%在尿液中。
Urine and feces were collected from mice fed labeled methoxychlor. Of recovered radioactivity, 90% was in feces and 10% in urine.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
(14C-1-苯基)-甲氧氯在油乳剂中给予大鼠静脉注射剂量为1毫克/0.5毫升乳剂时,被肝脏迅速代谢为未知的亲水性产物,这些产物主要通过粪便排出,通过肝脏分泌进入胆汁,以及较小程度上通过尿液排出。从胃肠道很少有再吸收发生。
(14C-1-Phenyl)-methoxychlor in oil emulsion given iv to rats at dose of 1 mg in 0.5 mL emulsion was metabolized rapidly by liver to unidentified hydrophilic products, which were excreted mainly in feces, by secretion from liver into bile, and to lesser extent in urine. Very little reabsorption occurred from GI tract.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
喂食含500毫克/公斤体重甲氧滴滴涕的断奶大鼠,在4到18周期间,在脂肪中储存了14-36毫克/公斤体重的甲氧滴滴涕。4周内达到平衡,停止暴露后2周内甲氧滴滴涕从脂肪组织中消失。喂食含100毫克/公斤体重的大鼠储存了1-7毫克/公斤体重。喂食含25毫克/公斤体重的大鼠没有储存甲氧滴滴涕,并且储存量没有性别差异。
Weanling rats fed 500 mg/kg diet methoxychlor for 4-18 wk stored 14-36 mg/kg in fat. Equilibrium was reached within 4 wk & methoxychlor disappeared from fatty tissue within 2 wk after end of exposure. Rats fed 100 mg/kg stored 1-7 mg/kg. No methoxychlor ... stored in animals given 25 mg/kg diet, & no sex differences in storage ... .
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:9
-
立即威胁生命和健康浓度:5,000 mg/m3
-
危险品标志:Xn,F,T,N
-
安全说明:S16,S23,S36/37/39,S45,S60,S61,S62,S7
-
危险类别码:R20/21/22
-
WGK Germany:3
-
危险品运输编号:UN 2811 6.1/PG 3
-
RTECS号:KJ3675000
-
海关编码:2909309011
-
储存条件:应将产品存放在阴凉、通风且干燥的地方,并确保不与食品、饲料或饮料等物品混放。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 甲氧滴滴涕;1,1,l-三氯-2,2-双(对甲氧苯基)乙烷 |
| 化学品英文名称: | Trichloro—2,2-bis(p-methoxyphenyl)ethane;Methoxychlor |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 72-43-5 |
| 分子式: | C 16 H 15 Cl 3 O 2 |
| 分子量: | 345.66 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:甲氧滴滴涕;1,1,l-三氯-2,2-双(对甲氧苯基)乙烷 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 |
| 健康危害: | 吸入、摄入或经皮肤吸收后对身体有害,具刺激作用。 |
| 环境危害: | 对环境有严重危害,对水体可造成污染。 |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解,放出有毒的烟气。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 灭火方法及灭火剂: | 在上风向灭火。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | 消防人员须佩戴防毒面具、穿全身消防服 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好口罩、护目镜,穿工作服。用大量水冲洗,经稀释的污水放入废水系统。如大量泄漏,收集回收或无害处理后废弃 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,局部排风。防止粉尘释放到车间空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防静电工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂、碱类接触。容器与传送设备要接地,防止产生静电。灌装时应控制流速,且有接地装置,防止静电积聚。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。包装密封。应与氧化剂、碱类分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:未制订标准美国TLV—TWA:10mg/m3 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 生产操作或农业使用时,佩戴防尘口罩。 |
| 眼睛防护: | 必要时可采用安全面罩。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作后,淋浴更衣。保持良好的卫生习惯 |
| 第九部分:理化特性 |
| 外观与性状: | 无色或白色粉末结晶。 |
| pH: | |
| 熔点(℃): | 77~89 |
| 沸点(℃): | (分解) |
| 相对密度(水=1): | 1.41(25℃) |
| 相对蒸气密度(空气=1): | 12 |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 16 H 15 Cl 3 O 2 |
| 分子量: | 345.66 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 不溶于水,易溶于芳烃。 |
| 主要用途: | 用作杀虫剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强碱。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:5000mg/kg(大鼠经口);7600mg/kg(大鼠经皮) LC50:无资料 |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 建议用焚烧法处置。在能利用的地方重复使用容器或在规定场所掩埋。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 无资料 |
| UN编号: | 无资料 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 无资料 |
| 运输注意事项: | 起运时包装要完整,装载应稳妥。运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、碱类、食用化学品等混装混运。运输途中应防曝晒、雨淋,防高温。运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。装运本品的车辆排气管须有阻火装置。中途停留时应远离火种、热源。车辆运输完毕应进行彻底清扫。公路运输时要按规定路线行驶。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 5 |
| MSDS修改日期: | 年月日 |
制备方法与用途
毒性
大鼠急性经口LD50>10000mg/kg(6000mg/kg),大鼠急性经皮LD50>10000mg/kg。对兔皮肤和眼睛有轻度刺激作用。大鼠经口30d无作用剂量为500mg/kg,2年喂养试验无作用剂量为200mg/kg。狗1年喂养试验无作用剂量为300mg/kg。动物试验未见致畸、致癌、致突变作用。野鸭LD50>2000mg/kg,鹌鹑>5000mg/kg,野鸡>5000mg/kg。虹鳟鱼LC50为45μg/L(24h),蜜蜂LD50为165.5μg/只。
化学性质原药为白色或乳黄色块状或片状固体,相对密度1.41。纯品熔点86~88℃,工业品约为77℃。易溶于苯、二甲苯等多种有机溶剂,能溶于乙醇和石油,难溶于水(0.1mg/L)。甲氧滴滴涕具有耐热性和抗氧化性,在醇碱溶液中比滴滴涕较难脱去氯化氢,但在重金属离子催化下,易脱去氯化氢。
用途有机氯杀虫剂。具有触杀和胃毒作用,无内吸作用,杀虫谱广,对人畜毒性较低,在有机体内无累积作用,易被多功能氧化酶分解,而转为水溶性无毒排泄物。可用于大田作物、果树、蔬菜等作物,防治玉米螟、造桥虫、豆甲、豌豆象、果树食心虫、苹果蠹蛾、日本金龟甲、小象虫、天幕毛虫、实蝇、叶蝉、蝽象、蔬菜叶跳甲、菜蛾、黄守瓜、种蝇等害虫,还可用于防治家畜体外寄生虫和卫生害虫。
生产方法在发烟硫酸或三氯化铝存在下,苯甲醚与三氯乙醛缩合制得甲氧滴滴涕。
类别农药
毒性分级中毒
急性毒性口服-大鼠 LD50: 1855毫克/公斤;口服-小鼠 LD50: 510毫克/公斤
可燃性危险特性燃烧产生有毒氯化物气体
储运特性库房通风低温干燥,与食品原料分开储运
灭火剂干粉、泡沫、砂土
职业标准TLV-TWA 10毫克/立方米;STEL 20毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1,1-三氯-2,2-二(4-羟基苯基)乙烷 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane 2971-36-0 C14H11Cl3O2 317.599 滴滴涕 p,p'-DDT 50-29-3 C14H9Cl5 354.49 2,2,2-三氯-1-(4-甲氧基苯基)乙醇 2,2,2-trichloro-1-(4-methoxyphenyl)ethan-1-ol 14337-31-6 C9H9Cl3O2 255.528 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— mono-OH-MXC 28463-03-8 C15H13Cl3O2 331.626 1,1-二氯-2,2-二(对甲氧基苯基)乙烷 1,1-dichloro-2,2-bis(p-methoxyphenyl)ethane 7388-31-0 C16H16Cl2O2 311.208 1,1,1-三氯-2,2-二(4-羟基苯基)乙烷 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane 2971-36-0 C14H11Cl3O2 317.599 —— 4,4-(2-chloroethane-1,1-diyl)bis(methoxybenzene) 4957-05-5 C16H17ClO2 276.763 1-甲氧基-4-[1-(4-甲氧基苯基)乙基]苯 1,1-bis(4-methoxyphenyl)ethane 10543-21-2 C16H18O2 242.318 1,1,4,4-四-(4-甲氧基-苯基)-丁烷 1,1,4,4-Tetra(p-methoxyphenyl)-butan 144211-22-3 C32H34O4 482.62 1-甲氧基-4-[1,2,2-三(4-甲氧基苯基)乙基]苯 1,1,2,2-tetrakis(4-methoxyphenyl)ethane 51048-43-2 C30H30O4 454.566 —— 2,2-bis-(4-methoxyphenyl)acetic acid 4541-73-5 C16H16O4 272.301 —— methyl ester of 2,2-bis(4,4'-dimethoxyphenyl)ethanoic acid 5359-41-1 C17H18O4 286.328 4,4-二甲氧基二苯基甲烷 4,4'-dianisylmethane 726-18-1 C15H16O2 228.291
反应信息
-
作为反应物:描述:甲氧滴滴涕 在 cyanocobalamin tris(2,2'-bipyridyl)ruthenium dichloride 作用下, 以 various solvent(s) 为溶剂, 生成 1,1-二氯-2,2-二(对甲氧基苯基)乙烷参考文献:名称:Recycled Catalysis of a Hydrophobic Vitamin B12in an Ionic Liquid摘要:利用离子液体作为反应介质,实现了在含有[Ru(II)(bpy)3]Cl2光敏剂的可见光照射系统中,疏水性维生素B12衍生物庚甲基氧钴因高氯酸盐在1,1-双(4-氯苯基)-2,2,2-三氯乙烷(DDT)脱氯反应中的循环利用。DOI:10.1246/cl.2005.1096
-
作为产物:参考文献:名称:Elbs, Journal fur praktische Chemie (Leipzig 1954), 1893, vol. <2> 47, p. 68摘要:DOI:
-
作为试剂:参考文献:名称:Baghos,V.B. et al., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1977, vol. 15B, p. 368 - 371摘要:DOI:
文献信息
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
表征谱图
-
氢谱1HNMR
-
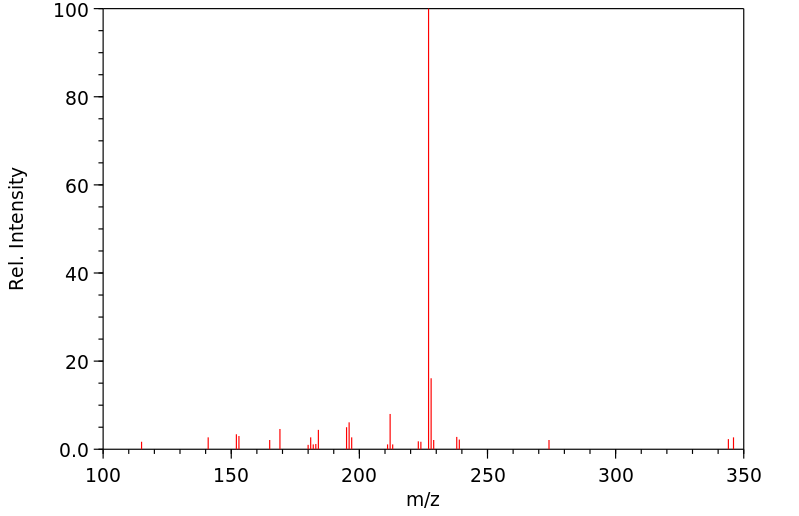
质谱MS
-
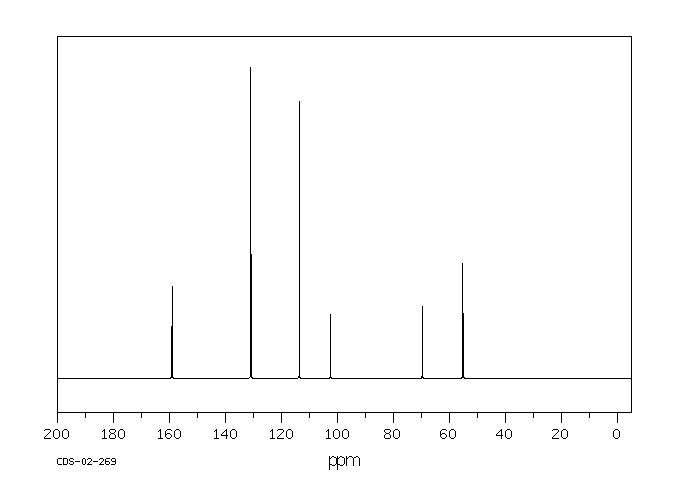
碳谱13CNMR
-
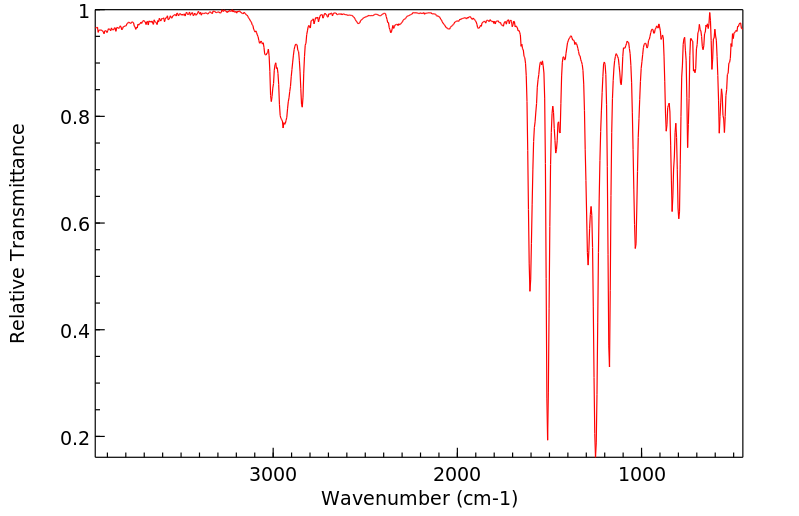
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫