滴滴涕 | 50-29-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:107-110 °C(lit.)
-
沸点:440.74°C (rough estimate)
-
密度:1.556 g/cm3
-
闪点:72 °C
-
溶解度:氯仿:微溶;甲醇:加热
-
暴露限值:NIOSH REL: 0.5 mg/m3, IDLH 500 mg/m3; OSHA PEL: TWA 1 mg/m3; ACGIH TLV: TWA 1 mg/m3.
-
物理描述:Ddt and metabolites appears as a colorless crystalline solid or white to off-white powder. Odorless to slightly aromatic. Insoluble in water. Used as an insecticide.
-
颜色/状态:Biaxial elongated tablets, needles from 95% alcohol
-
气味:Odorless or with slight aromatic odor
-
蒸汽压力:1.6X10-7 mm Hg at 20 °C
-
亨利常数:8.32e-06 atm-m3/mole
-
稳定性/保质期:
p,p'-DDT is dehydrochlorinated at temp above its mp to DDE, a reaction catalyzed by iron (III) or aluminum chlorides and UV light and, in soln, by alkali /or organic bases/. ... It is generally stable to oxidation ... Dehydrochlorination may occur above 50 °C.
-
分解:Decomp at 110 °C; dehydrochlorinates in alkali or org bases when in org solvents
-
腐蚀性:Should not be kept in iron containers
-
气味阈值:Detection threshold in water: 0.35 ppm
-
保留指数:2325.2;2263;2264;2270;2270;2277;2277;2264;2300;2289;2301;2330;2290;2284;2331.7;2280;2280.8;2291.9;2323.4;2335.7;2270;2325.6;2293;2290;2300;2300;2335;2306;2299;2290.7;2310.4
计算性质
-
辛醇/水分配系数(LogP):6.9
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:C
-
职业暴露限值:TWA: 0.5 mg/m3
-
危险等级:6.1(b)
-
立即威胁生命和健康浓度:500 mg/m3
-
危险品标志:O,T
-
安全说明:S16,S22,S26,S33,S36,S36/37,S45,S60,S61,S7
-
危险类别码:R52/53,R40,R36/37/38,R50/53,R11,R23/24/25,R39/23/24/25,R25,R48/25
-
WGK Germany:3
-
危险品运输编号:UN 2811 6.1/PG 3
-
RTECS号:KJ3325000
-
海关编码:2903920000
-
包装等级:III
-
危险类别:6.1(b)
-
储存条件:50%胶悬剂,每瓶装于2千克的塑料瓶中,并用纸箱外包装,每箱净重20千克。应避免与碱性物质接触,存放在阴凉处。包装需密封,严防火种。
制备方法与用途
化学性质
无色针状结晶。熔点为108.5-109℃,沸点260℃。易溶于吡啶及二氧六环。在100ml溶剂中的溶解度分别为:丙酮58g、四氯化碳45g、氯苯74g、乙醇2g、乙醚28g。不溶于水、稀酸和碱液。
用途
DDT曾是广泛使用的杀虫剂之一,具有胃毒和触杀作用,可加工成粉剂、乳剂或油剂使用。我国以前主要用于防治棉蕾铃期害虫、果树食心虫、农田作物粘虫、蔬菜菜青虫等,也用于环境卫生,防治蚊、蝇、臭虫等。然而,由于DDT不易被降解为无毒物质,在使用过程中容易造成积累并污染环境。残留于植物中的DDT可通过“食物链”或其他途径进入人和动物体内,沉积中毒,影响人体健康。目前,DDT已被禁止使用,但其一些工业用途仍需以DDT作为中间体,例如三氯杀螨醇。
用途
具有胃毒和触杀作用,属于高残留农药品种,用于防治多种昆虫和卫生害虫。
生产方法
由三氯乙醛与一氯化苯在发烟硫酸存在下缩合而得。具体步骤为:将三氯乙醛、氯苯投入缩合锅中,搅拌下滴加发烟硫酸。缩合后的物料静置分层,放净废酸后,用热水洗涤酸性滴滴涕氯苯溶液,并用氢氧化钠溶液进一步洗涤。然后蒸馏回收氯苯,残留物即熔融的滴滴涕冷却结晶得到成品。缩合反应在10-23℃进行,在此过程中同时加入缩合反应产生的废酸(含副反应生成的对氯苯磺酸)以抑制副反应并减少对氯苯磺酸的生成量。滴加硫酸的时间约2.5小时。一级品滴滴涕原粉中对位含量≥74.0%。
类别
农药
毒性分级
高毒
急性毒性
口服-大鼠 LD50: 87 毫克/公斤;口服-小鼠 LD50: 135 毫克/公斤
可燃性危险特性
受热分解生成有毒氯化物气体
储运特性
库房应保持通风、低温和干燥环境;与食品原料分开储存和运输
灭火剂
砂土、干粉、泡沫
职业标准
时间加权平均容许浓度(TWA):1 毫克/立方米;短时间接触极限值(STEL):3 毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三氯杀螨醇 Dicofol 115-32-2 C14H9Cl5O 370.49 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,2-双(对氯苯基)-1,1-二氯乙烷 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethane 72-54-8 C14H10Cl4 320.045 1-(4-氯苯基)-1-苯基-2,2-二氯乙烷 1,1-dichloro-2-(4-chlorophenyl)-2-phenylethane 6952-08-5 C14H11Cl3 285.6 1-氯-4-[2-氯-1-(4-氯苯基)乙基]苯 1-chloro-2,2-bis(p-chlorophenyl)ethane 2642-80-0 C14H11Cl3 285.6 1,1,1-三氯-2-(3-氯苯基)-2-(4-氯苯基)乙烷 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1,1-trichloroethane 4329-07-1 C14H9Cl5 354.49 —— 1,1-dichloro-2,2-diphenylethane 2387-16-8 C14H12Cl2 251.155 1-氯-4-[1-(4-氯苯基)乙基]苯 1,1-bis(4-chlorophenyl)ethane 3547-04-4 C14H12Cl2 251.155 1-氯-4-(1-苯基乙基)苯 1-chloro-4-(1-phenylethyl)benzene 60617-89-2 C14H13Cl 216.71 双(4-氯苯基)乙酰氯 bis(4-chlorophenyl)acetyl chloride 68668-89-3 C14H9Cl3O 299.584 氯化滴滴涕 1,1,1,2-tetrachloro-2,2-bis-(4-chloro-phenyl)-ethane 3563-45-9 C14H8Cl6 388.936 米托坦 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane 53-19-0 C14H10Cl4 320.045 三氯杀螨醇 Dicofol 115-32-2 C14H9Cl5O 370.49 甲氧滴滴涕 Methoxychlor 72-43-5 C16H15Cl3O2 345.653 1-氯-2-[2,2,2-三氯-1-(2-氯苯基)乙基]苯 1,1,1-trichloro-2,2-bis-(o-chlorophenyl)ethane 6734-84-5 C14H9Cl5 354.49 2,2-双(4-氯苯基)乙醛 2,2-bis(4-chlorophenyl)acetaldehyde 18164-50-6 C14H10Cl2O 265.139 2,2-双(4-氯苯基)乙醇 2,2-bis(p-chlorophenyl)ethanol 2642-82-2 C14H12Cl2O 267.155 1-氯-4-[1-(4-氯苯基)-2,2,2-三氟乙基]苯 1,1,1-trifluoro-2,2-bis(p-chlorophenyl)ethane 361-07-9 C14H9Cl2F3 305.127 —— 1,1-bis(4-chlorophenyl)propan-2-one 55525-14-9 C15H12Cl2O 279.166 双(4-氯苯基)乙酸 bis(4'-chlorophenyl)acetic acid 83-05-6 C14H10Cl2O2 281.138 —— (E)-1,1,4,4-tetrakis(4-chlorophenyl)-2,3-dichloro-2-butene 36954-67-3 C28H18Cl6 567.169 —— (Z)-1,1,4,4-tetrakis(4-chlorophenyl)-2,3-dichloro-2-butene 36954-66-2 C28H18Cl6 567.169 —— 1,1,4,4-tetrakis(4-chlorophenyl)-2,3-dichloro-2-butene 66291-82-5 C28H18Cl6 567.169 苯基乙苯 1,1'-ethylidenebis-benzene 612-00-0 C14H14 182.265 —— 2,2,3,3-tetrachloro-1,1,4,4-tetrakis-(4-chloro-phenyl)-butane 66291-83-6 C28H18Cl8 638.075 —— 1,1-bis-(4-chloro-phenyl)-acetone oxime 6941-84-0 C15H13Cl2NO 294.18 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Kunieda,T. et al., Chemical and pharmaceutical bulletin, 1977, vol. 25, p. 1749 - 1755摘要:DOI:
-
作为产物:参考文献:名称:Process for making 2,2-bis aryl trihaloethanes摘要:公开号:US02554269A1
-
作为试剂:参考文献:名称:真菌硫葡萄糖苷葡萄糖水解酶和十字花科植物表硫特异性蛋白的相互作用形成 1-氰基表硫烷烃:变构机制的影响摘要:摘要 通过来自真菌 Aspergillus sydowi QM 31c 的硫代葡萄糖苷葡萄糖水解酶和来自 Crambe abyssinica 的表硫特异性蛋白的相互作用,烯丙基硫代葡萄糖苷被转化为 1-氰基-2,3-环氧丙烷。所提供的动力学证据支持了这样的假设:epithiospecifier 蛋白以变构方式与硫葡糖苷葡萄糖水解酶相互作用。DOI:10.1016/s0031-9422(00)83521-9
文献信息
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
[EN] IMPROVED SYNTHETIC METHODS OF MAKING (2H-1,2,3-TRIAZOL-2-YL)PHENYL COMPOUNDS AS OREXIN RECEPTOR MODULATORS<br/>[FR] PROCÉDÉS SYNTHÉTIQUES AMÉLIORÉS POUR LA FABRICATION DE COMPOSÉS DE (2H-1,2,3-TRIAZOL-2-YL)PHÉNYLE UTILISÉS COMME MODULATEURS DES RÉCEPTEURS DE L'OREXINE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2021023843A1公开(公告)日:2021-02-11Processes for preparing (((3aR,6aS)-5-(4,6-dimethylpyrimidin-2-yl)hexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)(2-fluoro-6-(2H-l,2,3-triazol-2- yl)phenyl)methanone are described, which are useful for commercial manufacturing. Said compound is an orexin receptor modulator and may be useful in pharmaceutical compositions and methods for the treatment of diseased states, disorders, and conditions mediated by orexin activity, such as insomnia and depression.
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
[EN] SUBSTITUTED N-HETEROCYCLIC CARBOXAMIDES AS ACID CERAMIDASE INHIBITORS AND THEIR USE AS MEDICAMENTS<br/>[FR] CARBOXAMIDES N-HÉTÉROCYCLIQUES SUBSTITUÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA CÉRAMIDASE ACIDE ET LEUR UTILISATION EN TANT QUE MÉDICAMENTS申请人:BIAL BIOTECH INVEST INC公开号:WO2021055627A1公开(公告)日:2021-03-25The invention provides substituted N-heterocyclic carboxamides and related compounds, compositions containing such compounds, medical kits, and methods for using such compounds and compositions to treat a medical disorder, e.g., cancer, lysosomal storage disorder, neurodegenerative disorder, inflammatory disorder, in a patient.这项发明提供了替代的N-杂环羧酰胺和相关化合物,含有这些化合物的组合物,医疗工具包,以及使用这些化合物和组合物治疗患者的医疗疾病(例如癌症、溶酶体贮积症、神经退行性疾病、炎症性疾病)的方法。
-
[EN] CATHEPSIN CYSTEINE PROTEASE INHIBITORS<br/>[FR] INHIBITEURS DE PROTÉASES À CYSTÉINE DE TYPE CATHEPSINES申请人:MERCK SHARP & DOHME公开号:WO2015054038A1公开(公告)日:2015-04-16This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
表征谱图
-
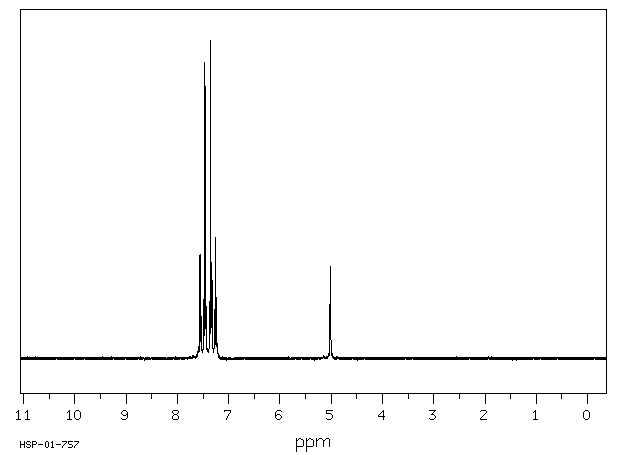
氢谱1HNMR
-
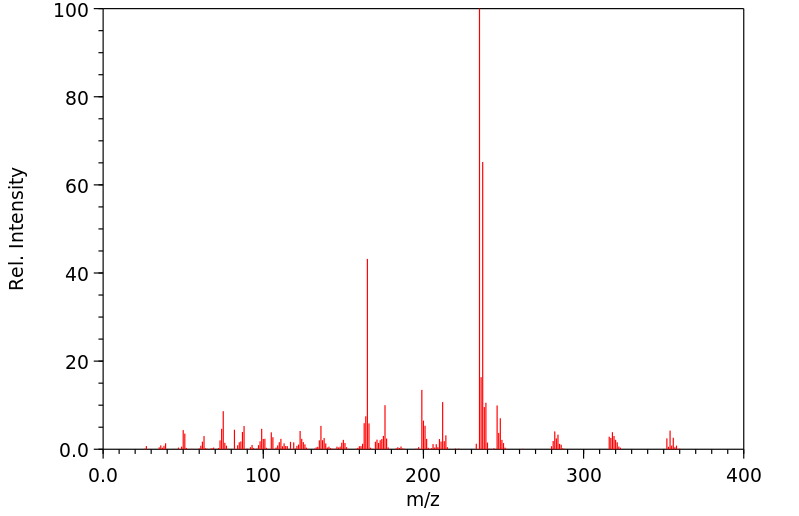
质谱MS
-
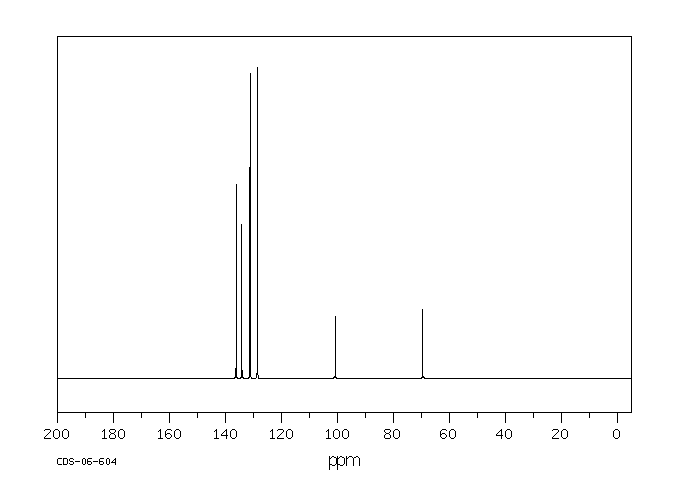
碳谱13CNMR
-
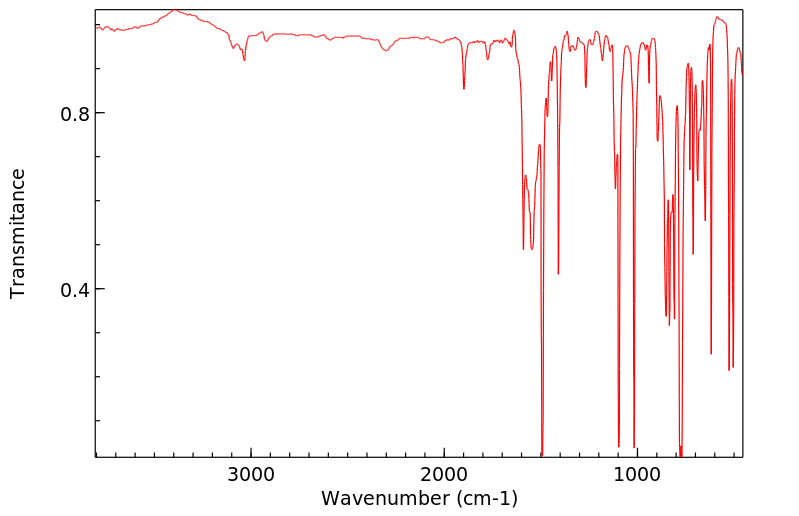
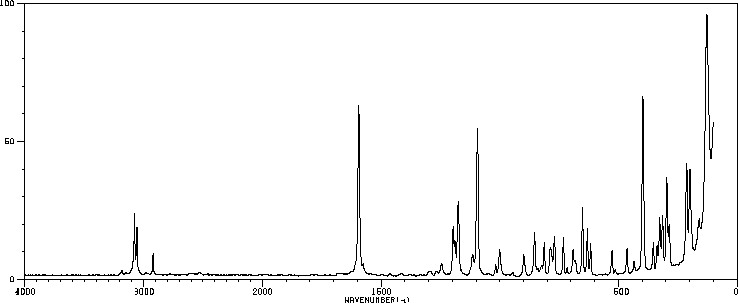
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息