5-氨基-4-氰基-3-甲基-1-苯基-1H-吡唑 | 5346-56-5
中文名称
5-氨基-4-氰基-3-甲基-1-苯基-1H-吡唑
中文别名
5-氨基-4-氰基-3-甲基-1-苯甲基吡唑
英文名称
5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile
英文别名
1-phenyl-3-methyl-4-cyano-5-aminopyrazole;5-amino-3-methyl-1-phenylpyrazole-4-carbonitrile
CAS
5346-56-5
化学式
C11H10N4
mdl
MFCD00128285
分子量
198.227
InChiKey
VWOYPUVIGCTVSC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:129 °C
-
沸点:408.2±45.0 °C(Predicted)
-
密度:1.23±0.1 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:67.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
海关编码:2933199090
-
储存条件:请将药品存放在避光、阴凉干燥的地方,并密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 5-Amino-4-cyano-3-methyl-1-phenyl-1H-pyrazole
Synonyms: 5-Amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5-Amino-4-cyano-3-methyl-1-phenyl-1H-pyrazole
CAS number: 5346-56-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H10N4
Molecular weight: 198.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 5-Amino-4-cyano-3-methyl-1-phenyl-1H-pyrazole
Synonyms: 5-Amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5-Amino-4-cyano-3-methyl-1-phenyl-1H-pyrazole
CAS number: 5346-56-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H10N4
Molecular weight: 198.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde 52217-35-3 C11H11N3O 201.228 1H-吡唑-4-甲硫代酰胺,5-氨基-3-甲基-1-苯基- 5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carbothioamide 101309-49-3 C11H12N4S 232.309 5-叠氮基-3-甲基-1-苯基吡唑-4-甲醛 5-azido-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde 114362-54-8 C11H9N5O 227.225 5-氯-3-甲基-1-苯基吡唑-4-甲醛 5-chloro-4-formyl-3-methyl-1-phenyl-1H-pyrazole 947-95-5 C11H9ClN2O 220.658 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl (E)-N-(4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)formimidate —— C14H14N4O 254.291 —— 3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile 18093-92-0 C11H9N3 183.213 —— 5-(N-methoxycarbonylamino)-4-cyano-3-methyl-1-phenyl-(1H)-pyrazole 194543-23-2 C13H12N4O2 256.264 —— 1-phenyl-3-methyl-4-carbamoyl-5-aminopyrazole 50427-81-1 C11H12N4O 216.242 1H-吡唑-4-甲硫代酰胺,5-氨基-3-甲基-1-苯基- 5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carbothioamide 101309-49-3 C11H12N4S 232.309 5-氨基-3-甲基-1-苯基吡唑 1-Phenyl-3-methyl-5-aminopyrazole 1131-18-6 C10H11N3 173.217 —— 5-((4-hydroxy-3-nitrobenzyl)amino)-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile 1448812-24-5 C18H15N5O3 349.349 —— N-(3-chloro-5-(((4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino)methyl)phenyl)propionamide 1448812-14-3 C21H20ClN5O 393.876 —— N-(5-(((4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino)methyl)-2-hydroxyphenyl)propionamide 1448812-16-5 C21H21N5O2 375.43 —— N-(5-(((4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino)methyl)-2-hydroxy-3-methylphenyl)propionamide 1448812-17-6 C22H23N5O2 389.457 —— 3-chloro-5-(((4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino)methyl)-N-ethyl-2-hydroxybenzamide 1448812-21-2 C21H20ClN5O2 409.875 —— 5-((3-chloro-4-hydroxy-5-nitrobenzyl)amino)-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile 1448812-26-7 C18H14ClN5O3 383.794 —— N-(3-chloro-5-(((4-cyano-3-methyl-1-phenyl-1H-pyrazole-5-yl)amino)methyl)-2-hydroxyphenyl)acetamide 1448812-18-7 C20H18ClN5O2 395.848 —— N-[3-chloro-5-[[(4-cyano-5-methyl-2-phenyl-pyrazol-3-yl)amino]methyl]-2-hydroxy-phenyl]butanamide 1448812-19-8 C22H22ClN5O2 423.902 —— 5-(3-chloro-4-methoxy-5-nitrobenzyl)amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile 1448812-23-4 C19H16ClN5O3 397.821 —— 1-Phenyl-3-methyl-4-pyrazolethiocarboxamide 137576-87-5 C11H11N3S 217.294 —— N-[3-chloro-5-[[(4-cyano-5-methyl-2-phenyl-pyrazol-3-yl)amino]methyl]-2-hydroxy-phenyl]hexanamide 1448812-20-1 C24H26ClN5O2 451.956 —— N-(5-(((4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino)methyl)-2-methoxy-3-chlorophenyl)propionamide 1448812-15-4 C22H22ClN5O2 423.902 —— 1-(4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)-3-(2-(2,2,2-trifluoroethyl)-1,2,3,4-tetrahydroisoquinolin-4-yl)urea 1610346-38-7 C23H21F3N6O 454.455 - 1
- 2
反应信息
-
作为反应物:描述:5-氨基-4-氰基-3-甲基-1-苯基-1H-吡唑 在 4-二甲氨基吡啶 、 硫酸 、 三乙胺 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 生成 3,6-dimethyl-1-phenyl-1,5-dihydro-4H-pyrazolo-[3,4-d]pyrimidin-4-one参考文献:名称:吡唑并嘧啶酮作为西地那非衍生物和核黄素的合成摘要:摘要 从各种吡唑酰胺(2a-d)合成了一系列新型吡唑并嘧啶酮衍生物(3(a-d)、4(a-d)和6(a-d)) 5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (1) 和各种氨基锂。此外,我们还描述了从简单的2-硝基苯甲酸以高产率合成具有喹唑啉部分的硬化素药物分子。图形概要DOI:10.1080/00397911.2018.1459720
-
作为产物:参考文献:名称:新型融合的吡唑并[4,3-d]吡啶和吡唑并[3,4-b] [1,8]萘啶异构体的设计,合成及药理作用:一类新型的有效和选择性乙酰胆碱酯酶抑制剂。摘要:一个新的他克林(THA)类似物家族(7-9,12),包含氮杂杂环吡唑并[4,3-d]吡啶或吡唑并[3,4-b] [1,8]萘啶系统,是喹啉的等排体THA环已合成。使用Ellman方法在大鼠脑胆碱酯酶中测试了这些化合物,所有这些化合物均能有效抑制该酶。化合物9和12b对乙酰胆碱酯酶(AChE)最有效,显示IC(50)为6.0和6.4 microM,对大鼠脑丁酰胆碱酯酶的活性较低,分别显示选择性指数5.3和20.9。还使用培养的大鼠皮层细胞在体外测试了化合物7-9和12的急性神经毒性。化合物7和8没有明显毒性;9在500 microM时有毒,但在100 microM时无毒。萘啶衍生物12a和12b表现出明显的浓度依赖性神经毒性,能够杀死500 microM的大多数细胞。使用来自加州鱼雷的AChE的X射线晶体结构的分子动力学模拟来解释这些新的THA等位基因的可能结合方式。DOI:10.1021/jm020391n
文献信息
-
[EN] BICYCLIC HETEROARYL UREA, THIOUREA, GUANIDINE AND CYANOGUANIDINE COMPOUNDS AS TRKA KINASE INHIBITORS<br/>[FR] COMPOSÉS D'HÉTÉROARYLE URÉE, THIOURÉE,GUANIDINE ET CYANOGUANIDINE EN TANT QU'INHIBITEURS DE LA KINASE TRKA申请人:ARRAY BIOPHARMA INC公开号:WO2014078408A1公开(公告)日:2014-05-22Compounds of Formula I or stereoisomers, tautomers, or pharmaceutically acceptable salts, solvates or prodrugs thereof, wherein Ring A, Ring C and X are as defined herein, are inhibitors of TrkA kinase and are useful in the treatment of diseases which can be treated with a TrkA kinase inhibitor such as pain, cancer, inflammation/inflammatory diseases, neurodegenerative diseases, certain infectious diseases, Sjogren's syndrome, endometriosis, diabetic peripheral neuropathy, prostatitis and pelvic pain syndrome.
-
Synthesis of New Pyrazolopyrimidinedithiones and Pyrazolopyrimidinephosphines from Aminocyanopyrazoles作者:Fatma Allouche、Fahker Chabchoub、Mansour Salem、Gilbert KirschDOI:10.1080/00397911.2010.486516日期:2011.4.19Abstract A general, high-yielding synthetic protocol for the expedited synthesis of functionalized pyrazolopyrimidine dithione 2 and pyrazolodiazaphosphininethione 3 from amino-cyano pyrazole 1 precursors is presented.
-
Synthesis of aminocyanopyrazoles via a multi-component reaction and anti-carbonic anhydrase inhibitory activity of their sulfamide derivatives against cytosolic and transmembrane isoforms作者:Fatma Allouche、Fakher Chabchoub、Fabrizio Carta、Claudiu T. SupuranDOI:10.3109/14756366.2012.720573日期:2013.4.1were converted to the corresponding sulfamides by reaction with sulfamoyl chloride. The aminopyrazoles incorporating phenyl and tosyl moieties were tested as inhibitors of four carbonic anhydrase (CA, EC 4.2.1.1) isoforms, the human (h) hCA I, II, IX and XII. Many of them showed low micromolar or submicromolar inhibition of these enzymes. The corresponding sulfamides were low nanomolar CA inhibitors.
-
Synthesis and properties of sildenafil isostere作者:Ziqi Su、Qi Zhang、Qieqiang Zhao、Wenyi Liu、Tao Zhao、Huiping Wang、Jiarong Li、Juan XuDOI:10.1002/ardp.202100145日期:2021.10series of novel pyrazolo[3,4-d]pyrimidin-4-one derivatives were synthesized and evaluated for their anti-phosphodiesterase-5 (PDE-5) activity. A total of 28 compounds, containing alkyl and aryl groups at the 1-N and 3-C positions on the pyrazole ring, and also bearing different alkyl substituents on the piperazine ring were synthesized. Four compounds (4d, 5d, 6d, and 5o) were found to have better
-
[EN] N-(ARYLALKYL)-N'-PYRAZOLYL-UREA, THIOUREA, GUANIDINE AND CYANOGUANIDINE COMPOUNDS AS TRKA KINASE INHIBITORS<br/>[FR] COMPOSÉS DE N-(ARYLALKYLE)-N'-PYRAZOLYLE-URÉE, DE THIOURÉE, DE GUANIDINE ET DE CYANOGUANIDINE EN TANT QU'INHIBITEURS DE LA KINASE TRKA申请人:ARRAY BIOPHARMA INC公开号:WO2014078331A1公开(公告)日:2014-05-22Compounds of Formula I or stereoisomers, tautomers, or pharmaceutically acceptable salts, solvates or prodrugs thereof, wherein Ring A, Ring C, X, Ra, Rb, Rc, Rd and n are as defined herein, are inhibitors of TrkA kinase and are useful in the treatment of diseases which can be treated with a TrkA kinase inhibitor such as pain, cancer, inflammation/inflammatory diseases, neurodegenerative diseases, certain infectious diseases, Sjogren's syndrome, endometriosis, diabetic peripheral neuropathy, prostatitis or pelvic pain syndrome.
表征谱图
-
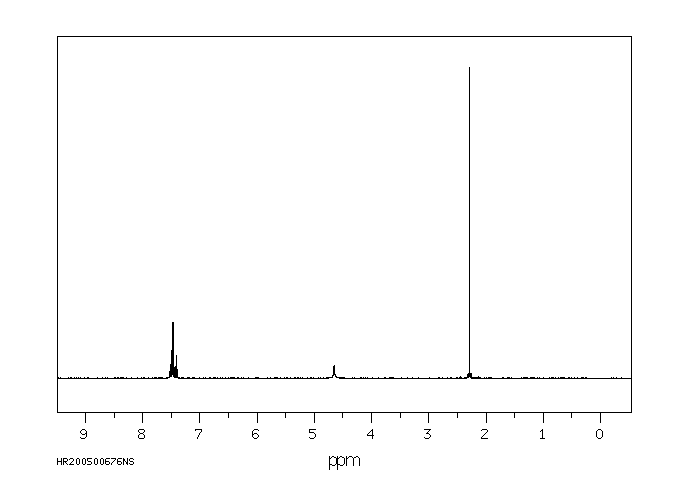
氢谱1HNMR
-
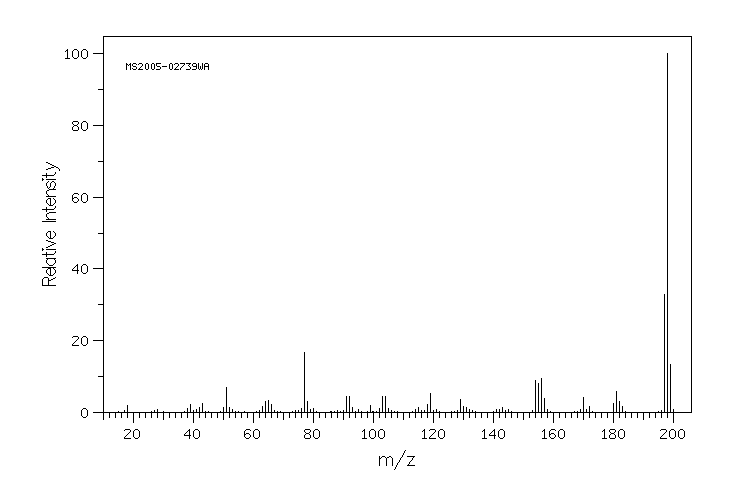
质谱MS
-
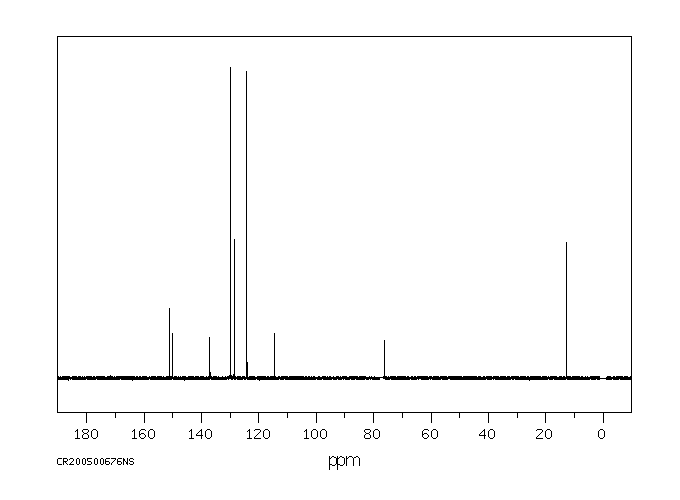
碳谱13CNMR
-
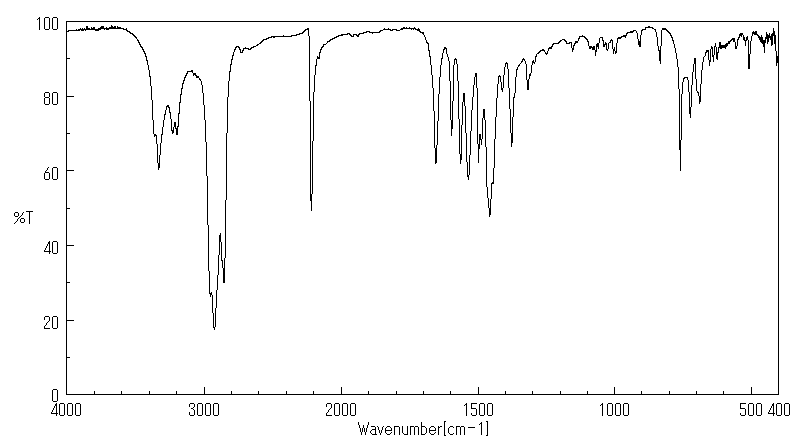
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)