4-氨基-2-氟苯酚 | 399-96-2
中文名称
4-氨基-2-氟苯酚
中文别名
3-氟-4-羟基苯胺;2-氟-4-氨基苯酚
英文名称
4-amino-2-fluorophenol
英文别名
2-fluoro-4-aminophenol;3-fluoro-4-hydroxyaniline;2-fluoro-4-aminphenol
CAS
399-96-2
化学式
C6H6FNO
mdl
——
分子量
127.118
InChiKey
MXJQJURZHQZLNN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:170-172°C
-
沸点:263.3±25.0 °C(Predicted)
-
密度:1.347±0.06 g/cm3(Predicted)
-
溶解度:可溶于二甲基亚砜、甲醇
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:46.2
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22
-
海关编码:2907111000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
4-Amino-2-fluorophenol Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-Amino-2-fluorophenol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 4
Acute toxicity (Oral)
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Harmful if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
[Response]
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-Amino-2-fluorophenol
Percent: >98.0%(GC)(T)
399-96-2
CAS Number:
Synonyms: 3-Fluoro-4-hydroxyaniline
4-Amino-2-fluorophenol
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Formula: C6H6FNO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Take care as it may decompose upon combustion or in high temperatures to
Specific hazards:
generate poisonous fume.
Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
Specific methods:
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
4-Amino-2-fluorophenol
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Pale yellow - Greyish yellow red
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:164°C (dec.)
No data available
Boiling Point/Range:
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Soluble in : Methanol
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Conditions to avoid: Air-sensitive
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen fluoride
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
4-Amino-2-fluorophenol
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-Amino-2-fluorophenol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 4
Acute toxicity (Oral)
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Harmful if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
[Response]
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-Amino-2-fluorophenol
Percent: >98.0%(GC)(T)
399-96-2
CAS Number:
Synonyms: 3-Fluoro-4-hydroxyaniline
4-Amino-2-fluorophenol
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Formula: C6H6FNO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Take care as it may decompose upon combustion or in high temperatures to
Specific hazards:
generate poisonous fume.
Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
Specific methods:
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
4-Amino-2-fluorophenol
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Pale yellow - Greyish yellow red
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:164°C (dec.)
No data available
Boiling Point/Range:
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Soluble in : Methanol
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Conditions to avoid: Air-sensitive
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen fluoride
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
4-Amino-2-fluorophenol
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
4-氨基-2-氟苯酚可用作医药化学和有机合成中间体。在有机合成转化中,结构中的酚羟基和氨基都可以与烷基卤化物反应生成相应的烷基化产物。该化合物可用于消毒剂、环氧树脂以及防腐剂的合成。
制备将锡(1.65克,14毫摩尔)和相应量的苯酚前体化合物(10毫摩尔)加入到100毫升圆底烧瓶中,再添加搅拌棒和回流冷凝器。向反应混合物中加入水(24毫升)、浓盐酸(9毫升),并在回流状态下加热反应1.5小时。反应结束后冷却反应混合物,然后加入20毫升5M氢氧化钠溶液,使用乙酸乙酯(3次,每次50 cm³)萃取水相。用饱和碳酸氢钠水溶液和盐水洗涤合并的有机层,并用硫酸镁干燥。过滤去除硫酸镁固体后浓缩有机层至干即可得到目标产物4-氨基-2-氟苯酚。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氟-4-甲氧基苯胺 3-fluoro-4-methoxyaniline 366-99-4 C7H8FNO 141.145 4-氨基-3-氟苯酚 4-amino-3-fluorophenol 399-95-1 C6H6FNO 127.118 2-氟-4-硝基苯酚 2-fluoro-4-nitrophenol 403-19-0 C6H4FNO3 157.101 2-氟苯酚 2-fluorophenol 367-12-4 C6H5FO 112.104 2-氟-4-硝基苯甲醚 2-fluoro-1-methoxy-4-nitrobenzene 455-93-6 C7H6FNO3 171.128 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(N-methyl)amino-2-fluorophenol 115602-79-4 C7H8FNO 141.145 —— 4-azido-2-fluorophenol —— C6H4FN3O 153.116 —— 4-ethoxy-3-fluoroaniline 399-39-3 C8H10FNO 155.172 N-(3-氟-4-羟基苯基)-乙酰胺 N-(3-fluoro-4-hydroxyphenyl)acetamide 2045-39-8 C8H8FNO2 169.155 3-氟-2-甲氧基苯胺 3-fluoro-2-methoxyaniline 437-83-2 C7H8FNO 141.145 —— 3-fluoro-4-octyloxyaniline 187329-16-4 C14H22FNO 239.333 —— 2-((3-fluoro-4-hydroxyphenyl)amino)-2-methylpropanenitrile —— C10H11FN2O 194.209 —— 2-fluoro-4-[bis(2'-hydroxyethyl)amino]phenol 496916-57-5 C10H14FNO3 215.224 —— 2-fluoro-4-[bis(2'-chloroethyl)amino]phenol —— C10H12Cl2FNO 252.116 4-(苄氧基)-3-氟苯胺 4-(benzyloxy)-3-fluoroaniline 168268-00-6 C13H12FNO 217.243
反应信息
-
作为反应物:描述:参考文献:名称:Aymes, Daniel J.; Paris, Michel R., Bulletin de la Societe Chimique de France, 1980, vol. 2, # 3-4, p. 175 - 178摘要:DOI:
-
作为产物:描述:参考文献:名称:le Guyader,M., Bulletin de la Societe Chimique de France, 1966, p. 1848 - 1858摘要:DOI:
-
作为试剂:描述:4-氨基-2-氟苯酚 、 1-(4-氟苯基氨基甲酰基)环丙烷羧酸 在 4-氨基-2-氟苯酚 作用下, 以93.6的产率得到N-(3-fluoro-4-hydroxyphenyl)-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide参考文献:名称:一种酪氨酸激酶抑制剂Foretinib的制备方法摘要:本发明提供了一种络氨酸酶抑制剂Foretinib的制备方法,即1,1‑环丙基二羧酸二乙酯选择性水解后与对氟苯胺酰胺化得到如式4所示的化合物,水解后再与4‑氨基‑2‑氟苯酚酰胺化得到如式6所示的化合物,4‑氯‑6‑甲氧基‑7‑喹啉醇与N‑(3‑氯丙基)吗啉发生取代得到如式8所示的化合物,如式6所示的化合物与如式8所示的化合物取代后得到目标产物N‑[3‑氟‑4‑({6‑(甲基氧基)‑7‑[(3‑吗啉‑4‑基丙基)氧基]‑喹啉‑4‑基}氧基)苯基]‑N’‑(4‑氟苯基)环丙烷‑1,1‑二甲酰胺粗品,该粗品在乙醇/丙酮溶液中重结晶得到高纯度产品,总收率44‑55%。本发明方法过程简单易行,原料易得,总收率高,产品质量好,适合工业化生产。公开号:CN105218445B
文献信息
-
脲取代的芳环连二噁烷并喹唑啉或喹啉类化 合物、组合物及其应用
-
Substituted pteridinones as p90 ribosomal S6 protein kinase (RSK) inhibitors: A structure-activity study作者:Kimberly A. Casalvieri、Christopher J. Matheson、Donald S. Backos、Philip ReiganDOI:10.1016/j.bmc.2019.115303日期:2020.3evaluate the structural features of BI-D1870 that are required for RSK2 inhibition. We have identified inhibitors of RSK2 activity, evaluated their target engagement in cells, and measured their effect on cell viability and cytotoxicity in the MOLM-13 acute myeloid leukemia (AML) cell line. The results of our studies support that RSK2 inhibition can be achieved in MOLM-13 cells without potent cytotoxicityp90核糖体S6激酶2(RSK2)的活性已成为癌症治疗的引人注目的靶标,因为它在多种细胞过程(如细胞转化和增殖)的调节中起着重要作用。几种泛RSK抑制剂已被BI-D1870鉴定,假类似物LJH685和LJI308是最具选择性,最有效和最常用的小分子抑制剂。我们设计和合成了一系列的翼龙和嘧啶,以评估BI-D1870抑制RSK2所需的结构特征。我们已经确定了RSK2活性的抑制剂,评估了它们在细胞中的靶标参与度,并测量了它们对MOLM-13急性骨髓性白血病(AML)细胞系中细胞活力和细胞毒性的影响。我们的研究结果支持在MOLM-13细胞中实现RSK2抑制而没有有效的细胞毒性。这项研究的结构活性数据将用作开发新型RSK2抑制剂的平台。
-
Discovery of a Pyrimidinedione Derivative as a Potent and Orally Bioavailable Axl Inhibitor作者:Hefeng Zhang、Xia Peng、Yang Dai、Jingwei Shao、Yinchun Ji、Yiming Sun、Bo Liu、Xu Cheng、Jing Ai、Wenhu DuanDOI:10.1021/acs.jmedchem.0c02093日期:2021.4.8therapeutics. We used molecular modeling-assisted structural optimization starting with the low micromolar potency compound 9 to discover compound 13c, a highly potent and orally bioavailable Axl inhibitor. Selectivity profiling showed that 13c could inhibit the well-known oncogenic kinase Met with equal potency to its inhibition of Axl superfamily kinases. Compound 13c significantly inhibited cellular Axl and受体酪氨酸激酶 Axl 在促进癌症进展、转移和耐药性中发挥重要作用,并已被确定为抗癌治疗的有希望的靶点。我们从低微摩尔效力化合物9开始,使用分子建模辅助结构优化来发现化合物13c ,这是一种高效且可口服生物利用的 Axl 抑制剂。选择性分析表明, 13c可以抑制众所周知的致癌激酶 Met,其抑制 Axl 超家族激酶的效力相同。化合物13c显着抑制细胞Axl和Met信号传导,抑制Axl和Met驱动的细胞增殖,并抑制Gas6/Axl介导的癌细胞迁移或侵袭。此外, 13c在 Axl 驱动和 Met 驱动的肿瘤异种移植模型中表现出显着的抗肿瘤功效,在耐受良好的剂量下导致肿瘤停滞或消退。所有这些有利的数据使13c成为癌症治疗的有前途的候选药物。
-
[EN] SUBSTITUTED PIPERAZINE COMPOUNDS AND METHODS AND USE THEREOF<br/>[FR] COMPOSÉS PIPÉRAZINIQUES SUBSTITUÉS, PROCÉDÉS ET UTILISATION ASSOCIÉS申请人:SUNSHINE LAKE PHARMA CO LTD公开号:WO2015169180A1公开(公告)日:2015-11-12Provided herein are novel piperazine compounds acting as selective serotonin reuptake inhibitors and/or the 5-HT1A receptor agonists. The invention also relates to the methods of preparing the compound and pharmaceutical composition, and the use of treating central nervous system dysfunction in mammals especially in humans.
-
Highly Ligand-Controlled Regioselective Pd-Catalyzed Aminocarbonylation of Styrenes with Aminophenols作者:Tongyu Xu、Feng Sha、Howard AlperDOI:10.1021/jacs.6b03161日期:2016.5.25Achieving chemo- and regioselectivity simultaneously is challenging in organic synthesis. Transition metal-catalyzed reactions are effective in addressing this problem by the diverse ligand effect on the catalyst center. Ligand-controlled regioselective Pd-catalyzed carbonylation of styrenes with aminophenols was realized, chemoselectively affording amides. Using a combination of boronic acid and 5-chlorosalicylic同时实现化学选择性和区域选择性在有机合成中具有挑战性。过渡金属催化的反应通过对催化剂中心的多种配体效应有效地解决了这个问题。实现了配体控制的区域选择性 Pd 催化苯乙烯与氨基酚的羰基化,化学选择性地提供酰胺。使用硼酸和 5-氯水杨酸的组合作为添加剂,在乙腈中使用三(4-甲氧基苯基)膦(L3)以高收率和选择性获得直链酰胺,而在丁酮中以高收率和选择性获得支链酰胺通过将配体更改为 1,3,5,7-tetramethyl-2,4,8-trioxa-6-phenyl-6-phosphaadamantane (L5)。进一步的研究表明,配体的性质是区域选择性的关键。锥角和托尔曼电子参数 (TEP) 与反应性和区域选择性相关。对酸添加剂的研究表明,不同的酸作为质子源,相应的反离子有助于提高反应性和选择性。
表征谱图
-
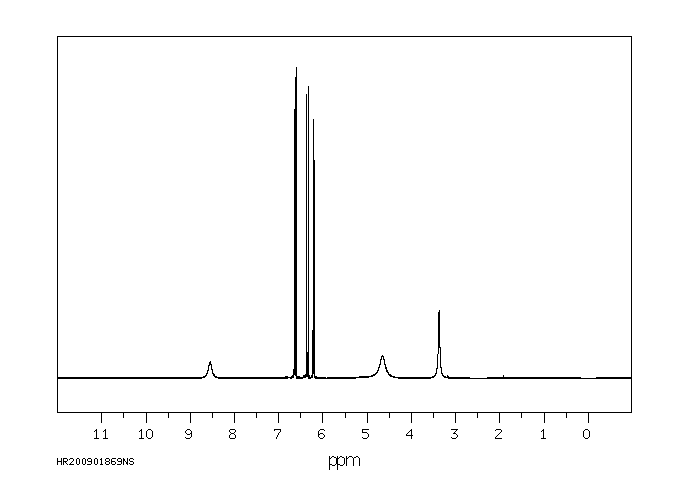
氢谱1HNMR
-
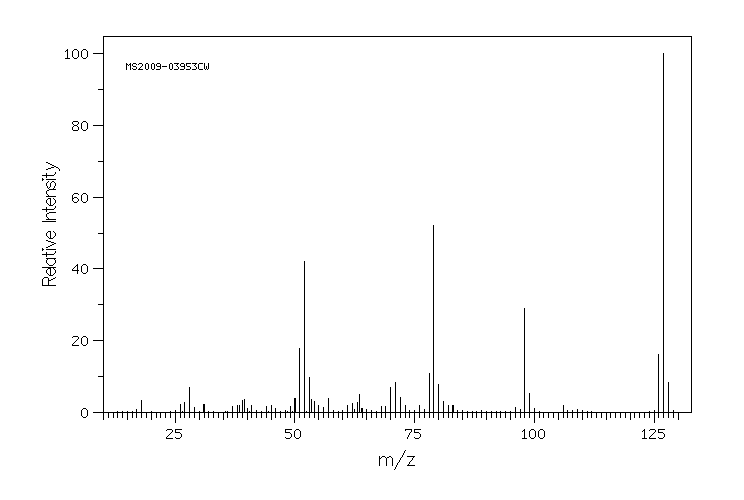
质谱MS
-
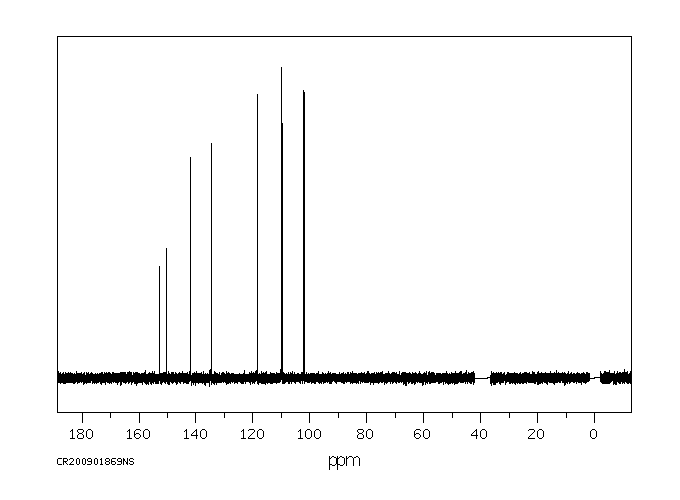
碳谱13CNMR
-
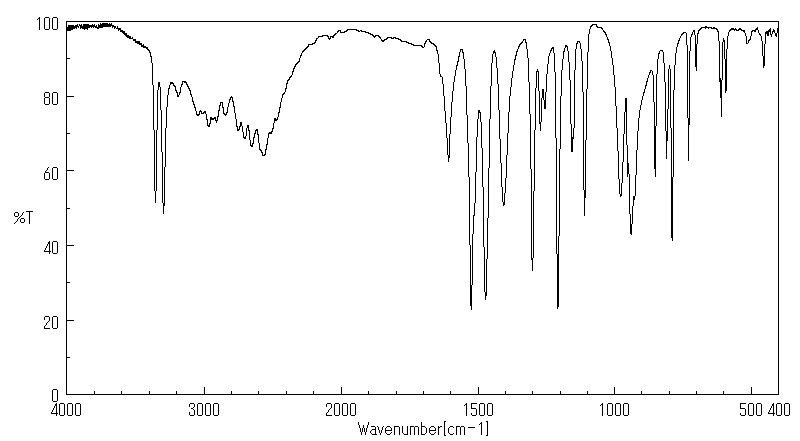
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚