苯甲醛肟 | 932-90-1
中文名称
苯甲醛肟
中文别名
苯亚甲基醛肟,PREDOMINANTLY(E)-ISOMER
英文名称
Benzaldoxime
英文别名
benzaldehyde oxime;α-benzaldoxime;N-benzylidenehydroxylamine
CAS
932-90-1
化学式
C7H7NO
mdl
——
分子量
121.139
InChiKey
VTWKXBJHBHYJBI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:30-33 °C
-
沸点:118-120°C 10mm
-
密度:1,11 g/cm3
-
闪点:108°C
-
溶解度:溶于甲醇,溶解度为0.1g/mL,澄清
-
介电常数:3.8(20℃)
-
稳定性/保质期:
在常温常压下稳定,应避免与氧化物、碱类、酸性氯化物及酸酐接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25,S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29280090
-
储存条件:请将容器密封保存,并存放在阴凉干燥处。
SDS
| Name: | Benzaldehyde oxime 99+% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 932-90-1 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 932-90-1 | Benzaldehyde oxime | 99.0+ | 213-261-2 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed. Prolonged contact may cause severe irritation or burns.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 932-90-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: after melting, clear colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 30.00 - 33.00 deg C
Autoignition Temperature: Not available.
Flash Point: 108 deg C ( 226.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H7NO
Molecular Weight: 121.14
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases, acid anhydrides, acid chlorides.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 932-90-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Benzaldehyde oxime - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 932-90-1: No information available.
Canada
CAS# 932-90-1 is listed on Canada's NDSL List.
CAS# 932-90-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 932-90-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:用作有机合成的试剂。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— aci-nitromethyl-benzene 622-43-5 C7H7NO2 137.138 —— benzylidenamine 16118-22-2 C7H7N 105.139 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— benzaldehyde chloroxime —— C7H6ClNO 155.584 —— O-isopropyl benzaldoxime 33499-41-1 C10H13NO 163.219 —— benzaldehyde O-allyl oxime 22892-77-9 C10H11NO 161.203 —— O-<2-Propyl>-benzaldoxim 22892-67-7 C10H9NO 159.188 —— (E)-benzaldehyde O-propargyloxime 283151-44-0 C10H9NO 159.188 —— O-tert-butyl benzaldoxime 942416-32-2 C11H15NO 177.246 —— benzohydroximoyl chloride 698-16-8 C7H6ClNO 155.584 —— phenylhydroxamoyl chloride 934-16-7 C7H6ClNO 155.584 O-(三甲基硅基)苯甲醛肟 O-(trimethylsilyl)benzaldoxime 17876-73-2 C10H15NOSi 193.321 苯甲酰胺肟 Benzamidoxime 613-92-3 C7H8N2O 136.153 苯甲醛腙 benzaldehyde, hydrazone 5281-18-5 C7H8N2 120.154 —— O-Acetylbenzaldoxim 19433-13-7 C9H9NO2 163.176 —— benzoxime O-(α-methyl)allyl ether 66013-16-9 C11H13NO 175.23 —— O-undecyl benzaldoxime —— C18H29NO 275.434 —— O-octyl benzaldehyde oxime 945260-70-8 C15H23NO 233.354 —— O-(benzyl)benzaldoxime 17146-21-3 C14H13NO 211.263 邻氯苯甲醛肟 2-chloro benzaldehyde oxime 3717-28-0 C7H6ClNO 155.584 —— O-methyl-benzamidoxime 4424-16-2 C8H10N2O 150.18 —— 1-benzylidene-2-methylhydrazine 13466-29-0 C8H10N2 134.181 —— benzaldehyde O-(methylsulphonyl)oxime —— C8H9NO3S 199.23 - 1
- 2
反应信息
-
作为反应物:描述:苯甲醛肟 在 potassium phosphate 、 N-氯代丁二酰亚胺 、 氧气 、 三乙胺 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 60.0 ℃ 、101.33 kPa 条件下, 反应 29.0h, 生成 3,5,5-triphenyl-4,5-dihydro-1,2,4-oxadiazole参考文献:名称:Orthogonal aerobic conversion of N-benzyl amidoximes to 1,2,4-oxadiazoles or quinazolinones摘要:通过在60°C下,在O2气氛中使用K3PO4作为催化剂,将N-苄基脒肟在DMF中加热,实现了1,2,4-噁二唑的简洁合成,这一过程涉及苄基C-H氧合作用。另一方面,在100°C下,使用Cs2CO3作为催化剂,在DMSO中对N-苄基脒脟进行需氧处理,可能导致骨架氧化重排,主要产物为喹唑啉酮。这种正交的产品选择性可以通过反应温度、溶剂和无机碱的选择来实现。DOI:10.1039/c3ob41393d
-
作为产物:描述:参考文献:名称:关于邻碘氧苯甲酸(IBX)氧化羟胺摘要:作为合成中碘的特别战略现代战略的一部分出版 抽象的 邻碘氧苯甲酸(IBX)被证实是氧化羟胺的有力工具。该合成路线被证明是有效和用户友好的,并且被用于各种碳水化合物衍生的N,N-二取代羟胺(环状,无环和官能化的),从而以良好的产率和区域选择性提供了相应的硝酮。N-单取代的羟胺根据反应条件显示出有趣的发散行为。据报道,IBX在二甲基亚砜中于45°C氧化可提供肟,而在室温下于二氯甲烷中的氧化则可有效提供相应的亚硝基二聚体。 邻碘氧苯甲酸(IBX)被证实是氧化羟胺的有力工具。该合成路线被证明是有效和用户友好的,并且被用于各种碳水化合物衍生的N,N-二取代羟胺(环状,无环和官能化的),从而以良好的产率和区域选择性提供了相应的硝酮。N-单取代的羟胺根据反应条件显示出有趣的发散行为。据报道,IBX在二甲基亚砜中于45°C氧化可提供肟,而在室温下于二氯甲烷中的氧化则可有效提供相应的亚硝基二聚体。DOI:10.1055/s-0036-1588457
-
作为试剂:描述:间溴氟苯 在 copper(l) iodide 、 N1-(4-Methoxybenzyl)-N2-(2-methyl-6-(5-methyl-1,3,4-oxadiazol-2-yl)phenyl)oxalamide 、 caesium carbonate 、 苯甲醛肟 作用下, 以 二甲基亚砜 为溶剂, 反应 18.0h, 生成 3-氟苯酚参考文献:名称:轻度铜催化羟基化反应发展的试剂设计和配体演化摘要:模块化配体库的并行合成和质量导向纯化,高通量实验和合理的配体演化,已经导致了一种新型的铜催化剂,可用于合成具有无痕氢氧化物的苯酚。此处报道的温和的反应条件使许多复杂的类药物酚的后期合成成为可能。DOI:10.1021/acs.orglett.7b01403
文献信息
-
Diversity Oriented Clicking (DOC): Divergent Synthesis of SuFExable Pharmacophores from 2‐Substituted‐Alkynyl‐1‐Sulfonyl Fluoride (SASF) Hubs作者:Christopher J. Smedley、Gencheng Li、Andrew S. Barrow、Timothy L. Gialelis、Marie‐Claire Giel、Alessandra Ottonello、Yunfei Cheng、Seiya Kitamura、Dennis W. Wolan、K. Barry Sharpless、John E. MosesDOI:10.1002/anie.202003219日期:2020.7.20Diversity Oriented Clicking (DOC) is a unified click‐approach for the modular synthesis of lead‐like structures through application of the wide family of click transformations. DOC evolved from the concept of achieving “diversity with ease” , by combining classic C−C π‐bond click chemistry with recent developments in connective SuFEx‐technologies. We showcase 2‐Substituted‐Alkynyl‐1‐Sulfonyl Fluorides面向多样性的点击 (DOC) 是一种统一的点击方法,用于通过应用广泛的点击转换家族来模块化合成类先导结构。DOC 从实现“轻松实现多样性”的概念演变而来,将经典的 C−C π 键点击化学与连接性 SuFEx 技术的最新发展相结合。我们展示了 2-取代的- A炔基-1-磺酰基F氟化物(SASFs)作为一类新的连接枢纽,与多种点击环加成过程相结合。通过具有一系列偶极子和环状二烯的 SASF 的选择性 DOC,我们以最少的合成步骤报告了 173 种独特功能分子的多样化点击库。SuFExable 库包含 10 个离散的杂环核心结构,这些核心结构源自 1,3- 和 1,5- 偶极子;而与环状二烯的反应会产生几种三维双环 Diels-Alder 加合物。通过对 96 孔板中的磺酰氟侧基进行 SuFEx 点击衍生化,可以通过后期修饰将文库增加到 278 种离散化合物——证明了 DOC 方法在快速合成不同功能结构方面的多功能性。
-
Terminal Alkyne-Assisted One-Pot Synthesis of Arylamidines: Carbon Source of the Amidine Group from Oxime Chlorides作者:Fengping Yi、Qihui Sun、Jing Sun、Chao Fu、Weiyin YiDOI:10.1021/acs.joc.9b00538日期:2019.6.7a diverse range of arylamidines from a novel cascade reaction of in situ generated nitrile oxides, sulfonyl azides, terminal alkynes, and water by [3 + 2] cycloaddition and ring opening sequence was developed. The use of aryl oxime chlorides as the carbon source of the amidine group and the addition of water proved to be critical for the reaction. Moreover, terminal alkynes, which can lead to high yields
-
[2 + 2 + 1] Heteroannulation of Alkenes with Enynyl Benziodoxolones and Silver Nitrite Involving C≡C bond Oxidative Cleavage: Entry to 3-Aryl-Δ<sup>2</sup>-isoxazolines作者:Cheng-Yong Wang、Fan Teng、Yang Li、Jin-Heng LiDOI:10.1021/acs.orglett.0c01285日期:2020.6.5A copper-catalyzed [2 + 2 + 1] heteroannulation of alkenes with enynyl benziodoxolones and AgNO2 involving oxidative cleavage of the C≡C bond promoted by cooperative Zn(OTf)2, KOAc, and 4 Å MS for producing 3-aryl Δ2-isoxazolines is reported. Mechanistic studies indicate that AgNO2 serves as the N/O two-atom unit source, enabling the formation of three bonds through NO2 addition across the C≡C bond
-
Facile Conversion of Acetals to Nitriles.
-
On the mixed oxides-supported niobium catalyst towards benzylamine oxidation作者:Álisson Silva Granato、Gustavo S. Gonçalves de Carvalho、Carla G. Fonseca、Javier Adrio、Alexandre A. Leitão、Giovanni Wilson AmaranteDOI:10.1016/j.cattod.2020.08.011日期:2021.12synthesized and applied towards oxidation reactions of benzylamine derivatives. Under the optimized reaction conditions, the selectivity to oxime enhanced, leading to the main product with up to 72 %. Moreover, even α-substituted benzylamines were well tolerated and led to oximes in good isolated yields. It is important to mention; four equivalents of the harmless and inexpensive hydrogen peroxide were employed
表征谱图
-
氢谱1HNMR
-
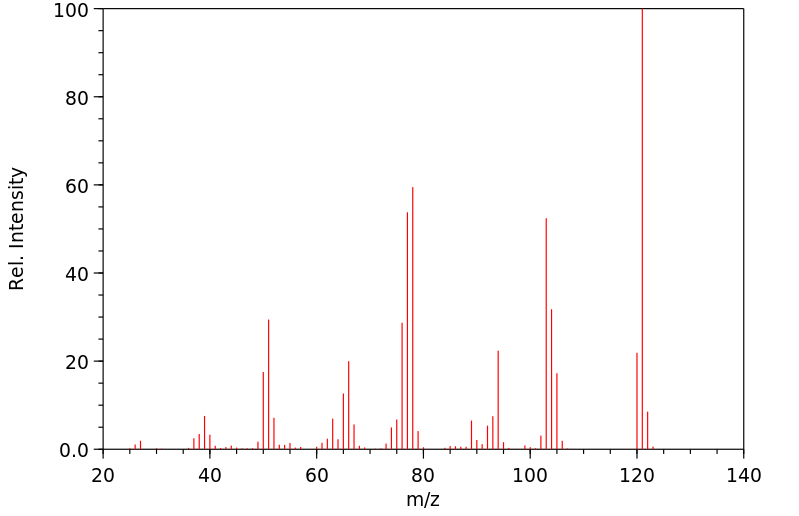
质谱MS
-
碳谱13CNMR
-
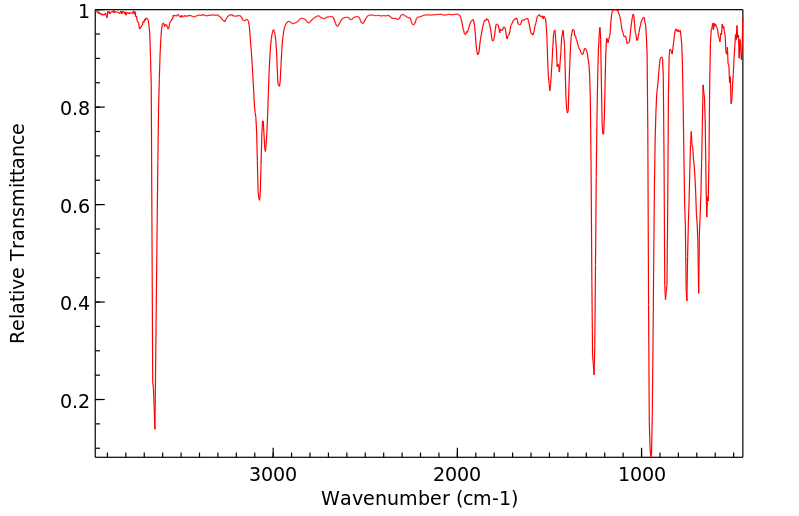
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫