苯甲醛肟 | 622-32-2
中文名称
苯甲醛肟
中文别名
苯亚甲基醛肟;(E)-苯甲醛肟;α-苯甲醛肟
英文名称
syn-benzaldehyde oxime
英文别名
benzaldehyde oxime;(E)-benzaldehyde oxime;(E)-N-(phenylmethylidene)hydroxylamine;benzaldoxime;(E)-benzaldoxime;(E)‐N‐(phenylmethylidene)hydroxylamine;(NE)-N-benzylidenehydroxylamine
CAS
622-32-2;932-90-1;622-31-1
化学式
C7H7NO
mdl
MFCD00002119
分子量
121.139
InChiKey
VTWKXBJHBHYJBI-SOFGYWHQSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:34-36 °C(lit.)
-
沸点:104 °C6 mm Hg(lit.)
-
密度:1.1111
-
闪点:228 °F
-
溶解度:溶于甲醇,溶解度为0.1g/mL,澄清
-
介电常数:3.7999999999999998
-
稳定性/保质期:
针状结晶在放置时会逐渐转化为E型,在加热或与稀酸作用下,这种转化过程会加快。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2928000090
-
储存条件:应存放在2-8°C的环境中,密闭、避光且保持通风干燥。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯甲醛肟 (Z)-Benzaldoxime 622-32-2 C7H7NO 121.139 —— benzylidenamine 16118-22-2 C7H7N 105.139 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯甲醛肟 (Z)-Benzaldoxime 622-32-2 C7H7NO 121.139 苯(甲)醛O-甲基肟 benzaldehyde-(O-methyl-seqcis-oxime ) 10229-54-6 C8H9NO 135.166 —— (E)-benzaldehyde O-methyloxime 10229-53-5 C8H9NO 135.166 —— O-Deutero-syn-benzaldoxim 14702-02-4 C7H7NO 122.131 —— benz-anti-aldoxim-ethyl ether 10229-56-8 C9H11NO 149.192 —— N-methyl-α-phenylnitrone 3376-23-6 C8H9NO 135.166 —— benzaldehyde O-allyloxime 50998-69-1 C10H11NO 161.203 —— 2-[(E)-benzylideneamino]oxyethanamine 21530-20-1 C9H12N2O 164.207 —— (E)-N-butoxy-1-phenylmethanimine 76129-34-5 C11H15NO 177.246 —— benzohydroximoyl chloride 698-16-8 C7H6ClNO 155.584 —— phenylhydroxamoyl chloride 934-16-7 C7H6ClNO 155.584 —— (E)-N-hydroxybenzimidoyl chloride 81745-44-0 C7H6ClNO 155.584 —— (E)-1-phenylethanone oxime 10341-75-0 C8H9NO 135.166 —— O-(trimethylsilyl)-E-benzaldoxime 103559-03-1 C10H15NOSi 193.321 苯甲醛腙 benzaldehyde, hydrazone 5281-18-5 C7H8N2 120.154 —— (E)-benzaldehyde hydrazone 41097-64-7 C7H8N2 120.154 —— (Z)-benzaldoxime O-acetate —— C9H9NO2 163.176 —— benzaldehyde imino-oxy acetic acid 141891-22-7 C9H9NO3 179.175 —— benzaldehyde O-(3-methylbut-2-enyl)oxime 217189-16-7 C12H15NO 189.257 —— benzaldehyde O-(1-methylallyl)oxime —— C11H13NO 175.23 —— (E)-3-(benzylideneaminoxy)propionic acid 103586-49-8 C10H11NO3 193.202 —— [(E)-benzylideneamino] N-methylcarbamate 140854-23-5 C9H10N2O2 178.191 邻氯苯甲醛肟 2-chloro benzaldehyde oxime 3717-28-0 C7H6ClNO 155.584 —— benzaldehyde-[(E)-O-(2-diethylamino-ethyl)-oxime ] 54631-88-8 C13H20N2O 220.315 —— benzaldehyde O-((E)-1-methylbut-2-enyl)oxime —— C12H15NO 189.257 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:在水中的有机反应。第1部分。使用锌粉还原亚胺的简便方法摘要:在温和条件下,在无任何有机溶剂的情况下,用锌粉在5%NaOH水溶液中还原亚胺,并以高收率获得相应的胺。DOI:10.1016/s0040-4039(98)01904-2
-
作为产物:参考文献:名称:新型高效催化体系,包括TEMPO /乙醛肟/ InCl 3,可将伯胺好氧氧化为肟摘要:描述了一种简单而有效的催化系统,该系统包括使用甲苯作为溶剂的TEMPO /乙醛肟/ InCl 3将伯胺有氧氧化为相应的肟。这种实用的方法可以使用O 2作为经济的绿色氧化剂,可耐受多种底物,从而可以以中等至极好的收率提供目标肟。DOI:10.1016/j.tetlet.2014.08.083
-
作为试剂:描述:5-溴-2-氯吡啶 在 苯甲醛肟 、 N1,N2-双(2-噻吩甲基)-乙二酰胺 、 caesium carbonate 、 copper(l) chloride 作用下, 以 二甲基亚砜 为溶剂, 反应 20.0h, 生成 2-羟基-5-溴吡啶参考文献:名称:从药物化合物库中鉴定铜催化的C-O偶联的草酰胺配体摘要:一个典型的药物化合物库具有分子多样性,可以为发现新的配体结构提供平台。本文中,我们描述了这种方法与高通量筛选的结合使用,以鉴定N,N'-双(噻吩-2-基甲基)草酰胺为配体,通常对铜催化的C-O交叉偶联有效。在温和的条件下都可以同时使用联芳醚和苯酚。DOI:10.1002/cctc.201900393
文献信息
-
[EN] TRICYCLIC HETEROCYCLIC COMPOUNDS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES TRICYCLIQUES申请人:BRISTOL MYERS SQUIBB CO公开号:WO2011059784A1公开(公告)日:2011-05-19Disclosed are compounds of Formula (I) or stereoisomers or salts thereof, wherein: X1, X2, X3, W, Q1, Q2, and G2 are defined herein. Also disclosed are methods of using such compounds as selective agonists for G protein-coupled receptor S1P1, and pharmaceutical compositions comprising such compounds. These compounds are useful in treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as autoimmune diseases and vascular disease.揭示了Formula (I)的化合物或其立体异构体或盐,其中:X1、X2、X3、W、Q1、Q2和G2在此处被定义。还揭示了将这些化合物用作G蛋白偶联受体S1P1的选择性激动剂的方法,以及包含这些化合物的药物组合物。这些化合物在治疗、预防或减缓多种治疗领域的疾病或疾病的进展方面是有用的,如自身免疫疾病和血管疾病。
-
Ruthenium-Catalyzed Rearrangement of Aldoximes to Primary Amides in Water作者:Rocío García-Álvarez、Alba E. Díaz-Álvarez、Javier Borge、Pascale Crochet、Victorio CadiernoDOI:10.1021/om3006917日期:2012.9.10The rearrangement of aldoximes to primary amides has been studied using the readily available arene-ruthenium(II) complex [RuCl2(η6-C6Me6)P(NMe2)3}] (5 mol %) as catalyst. Reactions proceeded cleanly in pure water at 100 °C without the assistance of any cocatalyst, affording the desired amides in high yields (70–90%) after short reaction times (1–7 h). The process was operative with both aromatic
-
Selective synthesis of E-isomers of aldoximes via a domino aza-Michael/retro-Michael reaction作者:Wei Chen、Wei-Guo Yu、Hai-Bo Shi、Xiao-Yan LuDOI:10.2478/s11696-012-0137-3日期:2012.1.1
Abstract A highly stereoselective synthesis of E-isomer of aldoximes was developed through a base-catalysed domino aza-Michael/retro-Michael reaction of hydroxylamine and 2-(R-benzylidene)malononitrile. This reaction generates (E)-aldoxime diastereomer in high yields (eight examples, isolated yields of 82-93 %), excellent diastereomeric purity (diastereomeric ratio higher than 95: 5 by 1H NMR), and proceeds under mild reaction conditions (aqueous NaOH, pH 12, room temperature, 4 h).
-
<i>m</i>-CPBA Mediated Metal Free, Rapid Oxidation of Aliphatic Amines to Oximes作者:Vilas V. Patil、Eknath M. Gayakwad、Ganapati S. ShankarlingDOI:10.1021/acs.joc.5b01740日期:2016.2.5An efficient, rapid oxidation of various aliphatic amines to oximes using m-CPBA as an oxidant in ethyl acetate is described. High conversion (100%) with >90% oxime selectivity is achieved at room temperature under catalyst-free conditions. Mild reaction conditions along with an easy work up procedure offer lower byproduct formation and high selectivity for oximes in good yield and purity.
-
Novel chemoenzymatic oxidation of amines into oximes based on hydrolase-catalysed peracid formation作者:Daniel Méndez-Sánchez、Iván Lavandera、Vicente Gotor、Vicente Gotor-FernándezDOI:10.1039/c7ob00374a日期:——chemoenzymatic process. This strategy is based on a two-step sequence developed in one-pot at 30 °C and atmospheric pressure. First, the formation of a reactive peracid intermediate occurs by means of a lipase-catalysed perhydrolysis reaction, and then this peracid acts as a chemical oxidising agent of the amines. A total of nine ketoximes were isolated in high purity after a simple extraction protocol (90–98%
表征谱图
-
氢谱1HNMR
-
质谱MS
-
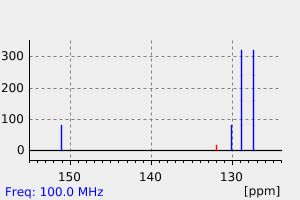
碳谱13CNMR
-
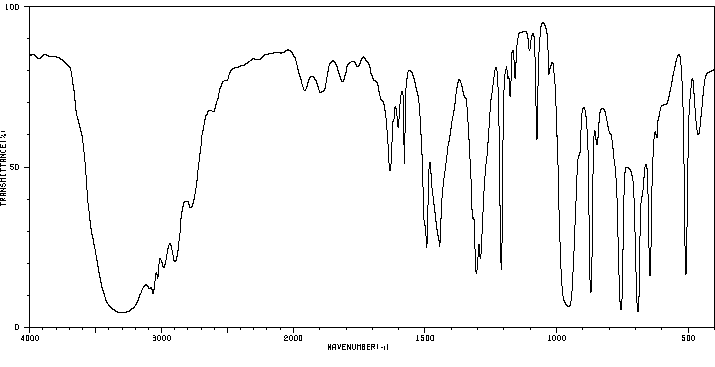
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫