3-甲基-6-(1-甲基乙亚基)环己烯 | 586-63-0
中文名称
3-甲基-6-(1-甲基乙亚基)环己烯
中文别名
——
英文名称
isoterpinolene
英文别名
p-mentha-2,4(8)-diene;2,4(8)-p-Menthadien;3-methyl-6-(1-methylethylidene)cyclohexene;(+/-)-Isoterpinolen;p-Menthadien-(2,4(8));cyclohexene, 3-methyl-6-(1-methylethylidene)-;Δ2,4(8)-p-Menthadien;2,4(8) p-menthadiene;3-methyl-6-propan-2-ylidenecyclohexene
CAS
586-63-0
化学式
C10H16
mdl
——
分子量
136.237
InChiKey
CIPXOBMYVWRNLL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:189 °C
-
密度:0.8561 g/cm3
-
LogP:4.450 (est)
-
保留指数:1064;1064;1073;1068;1067
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-异丙基-6-亚甲基-1-环己烯 beta-phellandrene 555-10-2 C10H16 136.237 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-异丙基-6-亚甲基-1-环己烯 beta-phellandrene 555-10-2 C10H16 136.237 —— (+/-)-2,4-p-menthadiene 586-68-5 C10H16 136.237
反应信息
-
作为反应物:参考文献:名称:Kabo, G. Ya.; Roganov, G. N.; Filippenko, Z. A., Russian Journal of Physical Chemistry, 1987, vol. 61, # 11, p. 1521 - 1522摘要:DOI:
-
作为产物:描述:参考文献:名称:Kabo, G. Ya.; Roganov, G. N.; Filippenko, Z. A., Russian Journal of Physical Chemistry, 1987, vol. 61, # 11, p. 1521 - 1522摘要:DOI:
文献信息
-
Terpene Cyclizations inside a Supramolecular Catalyst: Leaving-Group-Controlled Product Selectivity and Mechanistic Studies作者:Qi Zhang、Lorenzo Catti、Jürgen Pleiss、Konrad TiefenbacherDOI:10.1021/jacs.7b04480日期:2017.8.23mechanistic investigation was performed to elucidate the reaction mechanism. For the cyclization of geranyl acetate, it was found that the cleavage of the leaving group is the rate-determining step. Furthermore, the studies revealed that trace amounts of acid are required as cocatalyst. A series of control experiments demonstrate that a synergistic interplay between the supramolecular capsule and the从头到尾的萜烯环化可以说是自然界中最复杂的反应之一。氢键基间苯二甲烯胶囊代表了第一个能够催化该反应的人造酶样催化剂。基于胶囊和底物之间的非共价相互作用,可以通过使用不同的离去基团来调节产物的选择性。进行了详细的机理研究以阐明反应机理。对于乙酸香叶酯的环化,发现离去基团的裂解是决定速率的步骤。此外,研究表明需要痕量的酸作为助催化剂。一系列对照实验表明,超分子胶囊和酸痕迹之间的协同相互作用是催化活性所必需的。
-
Hybrid catalysts based on platinum and palladium nanoparticles for the hydrogenation of terpenes under slurry conditions作者:E. A. Karakhanov、M. P. Boronoev、E. S. Subbotina、A. V. Zolotukhina、A. L. Maximov、T. Yu. FilippovaDOI:10.1134/s0965544116120045日期:2016.12Catalysts based on platinum and palladium nanoparticles immobilized in mesoporous phenolformaldehyde polymers modified with sulfo groups have been used for the hydrogenation of a number of terpenes, such as (S)-(–)-limonene, α-terpinene, γ-terpinene, and terpinolene. It has been found that Pd-containing catalysts exhibit higher activity in the exhaustive hydrogenation of terpenes, whereas Pt-containing
-
Transition metal triflate catalyzed conversion of alcohols, ethers and esters to olefins作者:J. Keskiväli、A. Parviainen、K. Lagerblom、T. RepoDOI:10.1039/c8ra02437e日期:——efficient transition metal triflate catalyzed approach to convert biomass-based compounds, such as monoterpene alcohols, sugar alcohols, octyl acetate and tea tree oil, to their corresponding olefins in high yields. The reaction proceeds through C–O bond cleavage under solvent-free conditions, where the catalytic activity is determined by the oxophilicity and the Lewis acidity of the metal catalyst
-
Isomerization equilibrium of the p-menthadienes in the vapor phase作者:Z. A. Filippenko、O. M. Baranov、G. N. Roganov、G. Ya. KaboDOI:10.1007/bf00574247日期:——The isomerization equilibrium between nine p-menthadienes has been studied in the vapor phase at 250°C and their equilibrium ratios have been determined. A method for the quantitative GLC analysis of mixtures of isomeric p-menthadienes has been developed.
-
Thermal transformation of monoterpenes within thionin-supported zeolite Na-Y. Acid-catalyzed or electron transfer-induced?作者:Manolis Stratakis、Manolis Stavroulakis、Nikoletta SofikitiDOI:10.1002/poc.557日期:2003.1thionin-supported zeolite Na-Y. The same reactions occur in Na-Y dried under the same conditions as thionin/Na-Y. It is postulated that the thermal treatment of Na-Y generates ‘electron holes’ (probably acidic sites). The transformation of monoterpenes occurs more likely via an electron transfer-induced reaction subordinated to the occurrence of the acidic sites. The radical cation of the more thermodynamically
表征谱图
-
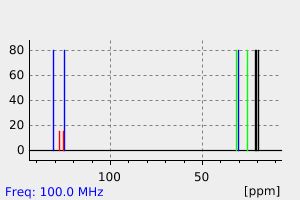
氢谱1HNMR
-
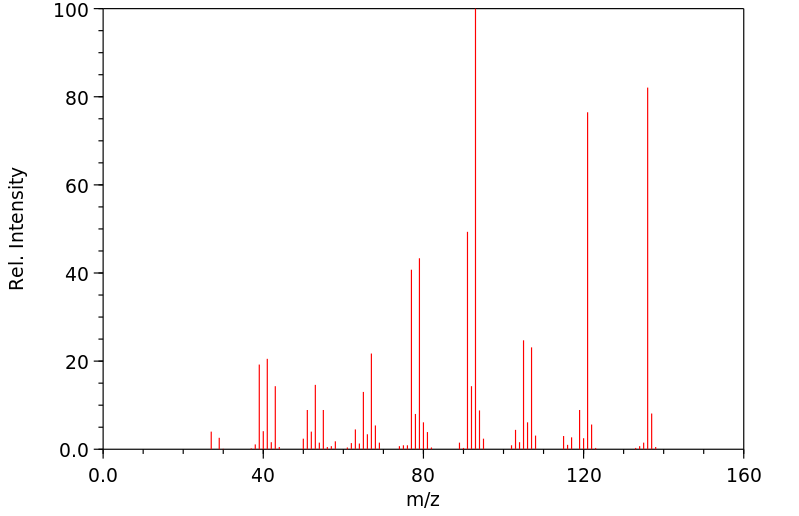
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸