硝基丙烷 | 108-03-2
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-108 °C
-
沸点:132 °C
-
密度:0.998 g/mL at 25 °C(lit.)
-
蒸气密度:3.1 (vs air)
-
闪点:93 °F
-
溶解度:14g/l
-
暴露限值:NIOSH REL: TWA 25 ppm (90 mg/m3), IDLH 1,000 ppm; OSHA PEL: TWA 25 ppm; ACGIH TLV: TWA 25 ppm (adopted).
-
介电常数:23.239999999999998
-
LogP:0.79 at 22℃
-
物理描述:1-nitropropane appears as a colorless oily flammable liquid. Density about the same as water. Vapors much heavier than air. Vapors may irritate skin, eyes and mucous membranes. Toxic oxides of nitrogen are released during combustion. Used as a propellant and as a solvent.
-
颜色/状态:Liquid
-
气味:Somewhat disagreeable odor
-
蒸汽密度:3.06 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.01X10+1 mm Hg at 25 °C /Extrapolated/
-
亨利常数:8.70e-05 atm-m3/mole
-
大气OH速率常数:4.40e-13 cm3/molecule*sec
-
稳定性/保质期:
Stable under recommended storage conditions.
-
自燃温度:421 °C
-
分解:Hazardous decomposition products formed under fire conditions: Carbon oxides, nitrogen oxides (NOx).
-
粘度:0.790 cP at 25 °C
-
燃烧热:481.363 Kcal/mol at 25 °C
-
汽化热:10.37 Kcal//mol at 25 °C
-
表面张力:30.64 dyne/cm at 20 °C
-
电离电位:10.81 eV
-
气味阈值:The AIHA Hygienic Guide states that the odor (of nitropropane) is detectable at 300 ppm but not at 80 ppm.
-
折光率:Index of refraction: 1.4018 at 20 °C/D
-
解离常数:pKa = 8.98
-
相对蒸发率:0.71 (Butyl acetate = 1)
-
保留指数:702.1;702.42;702.92;703.52;704.26;705.13;706.13;707.16;708.43;709.77;711.18;709.97;724;710;724;712;723;724;724;707;708;710;711;711;712;706;709;711;711;712;712;715;686;708;708;725;707;725
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 25 ppm (90 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:1,000 ppm
-
危险品标志:Xn
-
安全说明:S9
-
危险类别码:R20/21/22,R10
-
WGK Germany:1
-
海关编码:29042000
-
危险品运输编号:UN 2608 3/PG 3
-
危险类别:3
-
RTECS号:TZ5075000
-
包装等级:III
SDS
制备方法与用途
无色液体,带有类似氯仿的气味。熔点为-103.99℃,沸点为131.18℃,相对密度在20/4℃时为1.001,折射率为1.4016,闪点(闭杯)为49℃,燃点为419℃。与水共沸物中硝基丙烷含量为63.5%,共沸点为91.63℃。它能与空气形成爆炸性混合物,爆炸极限为2.6%(体积)。硝基丙烷能与醇、醚等有机溶剂混溶,并且微溶于水。
用途主要用作溶剂和中间体,喷气发动机燃料以及喷雾剂等。作为溶剂时,它对醇、酮、醚、酯、染料、油脂、蜡、树脂、合成橡胶均有很强的溶解力。与酒精并用是乙酸纤维的强力溶剂;与酒精、芒烃并用可代替氯化烃类溶剂溶解三乙酸纤维。作为低温溶剂可以溶解氯乙烯-乙酸乙烯的共聚物,还可用于溶解硝酸纤维。
此外,硝基丙烷还可用作胺类、羟胺类、硝基羟基化合物、氯化硝基烷烃等化工产品的中间体。例如,在硫酸存在下,硝基丙烷与甲烷水解可得硫酸羟胺和丙酸。在医药工业中用于生产抗结核药盐酸乙胺丁酯。
生产方法-
硝化丙烷:先将丙烷预热至430-450℃,然后引入内衬玻璃或二氧化硅的反应塔。向内部喷注75%硝酸,调节反应温度为390-440℃,压力为0.69-0.86MPa,并保持摩尔比为5:1。气体通过冷凝器冷却后,硝基丙烷与稀硝酸即被分离,而丙烷和气态氧化物则回收利用。该方法可得到硝基甲烷(10%-30%)、硝基乙烷(20%-25%)、1-硝基丙烷(25%)和2-硝基丙烷(40%)。此过程也可使用丙烯进行。
-
其他生产途径:可通过不饱和烃的气相或液相硝化,或者饱和烃的液相硝化制得硝基丙烷。
易燃液体
毒性分级高毒
急性毒性口服-大鼠 LD50: 455 毫克/公斤
刺激数据眼睛-人:150 ppm/15 分钟
爆炸物危险特性与空气混合可爆炸
可燃性危险特性易燃;高温下释放有毒氧化氮气体
储运特性库房需通风低温干燥,应与氧化剂分开储运
灭火剂干粉、二氧化碳、砂土
职业标准时间加权平均容许浓度(TWA)90 毫克/立方米;短时间接触容许浓度(STEL)150 毫克/立方米
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-bromo-3-nitropropane 16694-53-4 C3H6BrNO2 167.99 1-氯-3-硝基丙烷 1-chloro-3-nitropropane 16694-52-3 C3H6ClNO2 123.539 2-氯-1-硝基丙烷 2-chloro-1-nitropropane 503-76-4 C3H6ClNO2 123.539 3-硝基戊烷 3-nitropentane 551-88-2 C5H11NO2 117.148 1-溴-1-硝基丙烷 1-bromo-1-nitropropane 5447-96-1 C3H6BrNO2 167.99 1-氯-1-硝基丙烷 1-chloro-1-nitropropane 600-25-9 C3H6ClNO2 123.539 丙胲 N-propylhydroxylamine 627-38-3 C3H9NO 75.1106
反应信息
-
作为反应物:参考文献:名称:从硝基到羰基的新转化摘要:通过用碱和MoO 5 ·Pyr·HMPA处理将硝基化合物转化为相应的羰基化合物。DOI:10.1016/s0040-4039(01)92468-2
-
作为产物:参考文献:名称:Drug Therapy and Prevalence of Erectile Dysfunction in the Massachusetts Male Aging Study Cohort摘要:研究目标:检查常用药物与勃起功能障碍(ED)在两个时间点的关联。 设计:基于人群的横断面调查分析。 参与者:从马萨诸塞州男性衰老研究(MMAS)中随机选取的男性队列,包括1476名男性作为基线(1987-1989年)和922名男性用于后续分析(1995-1997年)。 干预:通过卡方统计分析特定药物类别之间的粗略关联。使用逻辑回归分析来区分药物效应与心脏病、高血压、未治疗糖尿病或抑郁症状的影响。 测量和主要结果:在MMAS中,通过家庭访问访谈收集病史、当前药物使用情况和勃起功能状态。在未调整分析中,噻嗪类和非噻嗪类利尿剂、β-阻滞剂、钙通道阻滞剂、Angiotensin转化酶抑制剂、苯二氮平、洋地黄、硝酸盐、3-羟基-3-甲基谷氨酰辅酶A还原酶抑制剂和组胺H2受体拮抗剂与已存在的ED相关。调整合并症和健康行为后,这些关联减弱,仅非噻嗪类利尿剂和苯二氮平的统计显著性仍然存在。 结论:一些常用药物可能增加ED的发生率;然而,需要来自更大人群的额外数据,以确定这些关联是否独立于潜在健康状况,并探索剂量和使用持续时间的影响。DOI:10.1592/phco.21.7.676.34571
-
作为试剂:参考文献:名称:Syntheses and cytotoxicity evaluation of bis(indolyl)thiazole, bis(indolyl)pyrazinone and bis(indolyl)pyrazine: analogues of cytotoxic marine bis(indole) alkaloid摘要:2,4-Bis(3'-indolyl)thiazoles, 3,5-bis(3'-indolyl)-2(1H)pyrazinone and 3,6-bis(3'-indolyl)pyrazine were synthesized and evaluated for cytotoxic activity against diverse human cancer cell lines by the National Cancer Institute. These compounds demonstrated significant inhibitory effects in the growth of a range of cancer cell lines. 2,4-Bis(3'-indolyl)thiazole displayed selective cytotoxicity against certain leukemia cell lines with GI(50) values in the low micromolar range while the substituted derivatives showed a broad spectrum of cytotoxic activity. 3,5-Bis(3'-indolyl)-2(1H)pyrazinone and 3,6-bis[3'-(N-methyl-indolyl)]pyrazine possessed strong inhibitory activity against a wide range of human tumor cell lines. The mechanism of action remained unknown. The results suggested that 2,4-bis(3'-indolyl)thiazoles, 3,5-bis(3'-indolyl)-2(1H)pyrazinone and 3,6-bis[3'-(N-methyl-indolyl)] pyrazine offer potential as lead compounds for the discovery of anticancer agents. (C) 2000 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0968-0896(99)00290-4
文献信息
-
A General Carbazole Synthesis via Stitching of Indole–Ynones with Nitromethanes: Application to Total Synthesis of Carbazomycin A, Calothrixin B, and Staurosporinone作者:Shweta Singh、Ramesh Samineni、Srihari Pabbaraja、Goverdhan MehtaDOI:10.1021/acs.orglett.9b01111日期:2019.5.3functionalized carbazole frameworks (28 examples). The scope of this new benzannulation has been extended to variants like 2-chloroindole-3-ynones to eventuate in chemo-differentiated 1,2,3,4-tetrasubstituted carbazoles with retention of the nitro group. The efficacy of this strategy has been demonstrated through concise total synthesis of natural products, viz. carbazomycin A, calothrixin B, and staurosporinone
-
[EN] BIS-HETEROARYL DERIVATIVES AS MODULATORS OF PROTEIN AGGREGATION<br/>[FR] DÉRIVÉS BIS-HÉTÉROARYLIQUES EN TANT QUE MODULATEURS DE L'AGRÉGATION DES PROTÉINES申请人:NEUROPORE THERAPIES INC公开号:WO2017020010A1公开(公告)日:2017-02-02The present invention relates to certain bis-heteroaryl compounds, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto- temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
-
Application of Silicon-Initiated Water Splitting for the Reduction of Organic Substrates作者:Ashot Gevorgyan、Satenik Mkrtchyan、Tatevik Grigoryan、Viktor O. IaroshenkoDOI:10.1002/cplu.201800131日期:2018.5several important classes of organic compounds is described. It is found that the reductive water splitting can be promoted by several metalloids among which silicon shows the best efficiency. The developed methodologies were applied for the reduction of nitro compounds, N-oxides, sulfoxides, alkenes, alkynes, hydrodehalogenation as well as for the gram-scale synthesis of several substrates of industrial
-
[EN] NOVEL DIHYDROQUINOLIZINONES FOR THE TREATMENT AND PROPHYLAXIS OF HEPATITIS B VIRUS INFECTION<br/>[FR] NOUVELLES DIHYDROQUINOLIZINONES POUR LE TRAITEMENT ET LA PROPHYLAXIE D'UNE INFECTION PAR LE VIRUS DE L'HÉPATITE B申请人:HOFFMANN LA ROCHE公开号:WO2015173164A1公开(公告)日:2015-11-19The invention provides novel compounds having the general formula: wherein R1, R2, R3, R4, R5, R6, X and Y are as described in the description and in the claims, as well as or pharmaceutically acceptable salts, or enantiomers, or diastereomers thereof. The invention also contains compositions including the compounds and methods of using the compounds.该发明提供了具有以下一般式的新化合物:其中R1、R2、R3、R4、R5、R6、X和Y如描述和权利要求中所述,以及其药学上可接受的盐,或对映体,或非对映异构体。该发明还包括包括这些化合物的组合物和使用这些化合物的方法。
-
One-pot synthesis of N-substituted pyrroles from nitro compounds and 2,5-hexadione over a heterogeneous cobalt catalyst作者:Zheng Gong、Yu Lei、Peng Zhou、Zehui ZhangDOI:10.1039/c7nj01898c日期:——study, the one-pot heterocyclization of nitro compounds with 2,5-hexadione was studied for the synthesis of N-substituted pyrroles via a Paal–Knorr condensation process. The heterogeneous cobalt–nitrogen catalyst (Co–Nx/C-800-AT) was found to be active for this reaction with formic acid. Formic acid served as a hydrogen donor for the transfer hydrogenation, and also acted as an acid catalyst. More importantly
表征谱图
-
氢谱1HNMR
-
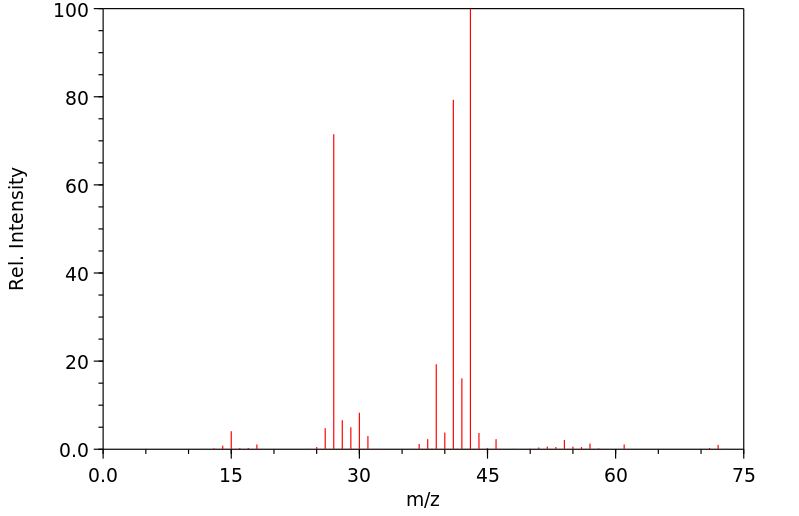
质谱MS
-
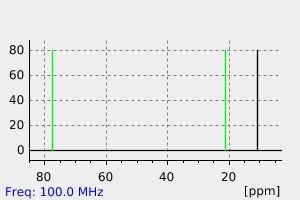
碳谱13CNMR
-
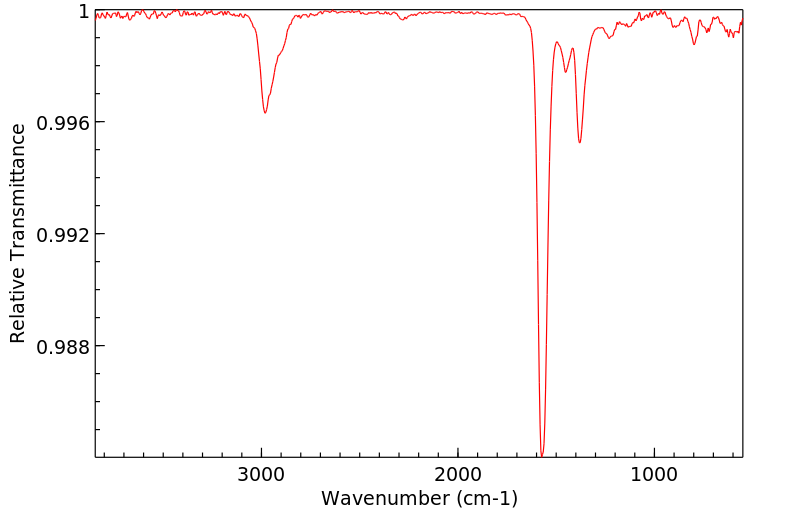
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息