1-(4-溴苯基)-4,4,4-三氟-1,3-丁二酮 | 18931-61-8
中文名称
1-(4-溴苯基)-4,4,4-三氟-1,3-丁二酮
中文别名
——
英文名称
4,4,4-trifluoro-1-(4-bromophenyl)butane-1,3-dione
英文别名
1-(4-bromophenyl)-4,4,4-trifluorobutane-1,3-dione;4-(p-bromophenyl)-1,1,1-trifluorobutane-2,4-dione
CAS
18931-61-8
化学式
C10H6BrF3O2
mdl
MFCD00510742
分子量
295.056
InChiKey
ITVIRNOCIDFYRZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:53-55°C
-
沸点:311.7±42.0 °C(Predicted)
-
密度:1.606±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:16
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2914700090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温且干燥环境中保存。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4,4,4-trifluoro-1-[4-(furan-2-yl)phenyl]butane-1,3-dione 252561-06-1 C14H9F3O3 282.219
反应信息
-
作为反应物:描述:1-(4-溴苯基)-4,4,4-三氟-1,3-丁二酮 在 Oxone 、 四(三苯基膦)钯 、 sodium carbonate 、 caesium carbonate 作用下, 以 甲醇 、 水 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 11.5h, 生成 乙基2-[5-[3'-(甲基磺酰基)联苯基-4-基]-3-(三氟甲基)-1H-吡唑-1-基]乙酸酯参考文献:名称:[EN] LIVER X RECEPTOR (LXR) MODULATORS FOR THE TREATMENT OF DERMAL DISEASES, DISORDERS AND CONDITIONS
[FR] MODULATEURS DU RÉCEPTEUR HÉPATIQUE X (LXR) PERMETTANT DE TRAITER LES MALADIES, TROUBLES ET AFFECTIONS DERMIQUES摘要:本文描述了肝X受体(LXR)调节剂以及在治疗皮肤疾病、紊乱或状况中利用LXR调节剂的方法。本文还描述了含有这些化合物的药物组合物。公开号:WO2013130892A1 -
作为产物:参考文献:名称:合成1-叔丁基-3(5)-三氟甲基-1H-吡唑的区域选择性和反应介质的比较研究摘要:介绍了从 4-烷氧基-1,1,1-三氟-3-烯烃-2 反应合成一系列 1-叔丁基-3(5)-(三氟甲基)-1H-吡唑的研究-ones [CF3C(O)CH=C(R1)(OR),其中 R = Et 且 R1 = H 或 R = Me 且 R1 = Me、Ph、4-Me-C6H4、4-MeO-C6H4、4- F-C6H4、4-Cl-C6H4、4-Br-C6H4、4-I-C6H4、fur-2-yl、thien-2-yl或naphth-2-yl]与叔丁基肼盐酸盐。当[BMIM][BF4](1-丁基-3-甲基咪唑四氟硼酸盐)和吡啶用作反应介质时,我们得到了1-叔丁基-3(5)-三氟甲基吡唑的混合物。当反应在乙醇中的 NaOH 中进行时,会形成具有高区域选择性的 5-三氟甲基-1-叔丁基-1H-吡唑。4-烷氧基-1,1水解后,生成1-叔丁基-3-三氟甲基-1H-吡唑,DOI:10.1002/ejoc.201201111
文献信息
-
Sulfamoylheteroaryl pyrazole compounds as anti-inflammatory/analgesic agents申请人:PFIZER INC.公开号:US20030144280A1公开(公告)日:2003-07-31This invention relates to a compound of the formula: 1 or a pharmaceutically acceptable salt thereof, wherein A and R 1 are each an optionally substituted 5 to 6-membered heteroaryl, wherein the heteroaryl is optionally fused to a carbocyclic ring or 5 to 6-heteroaryl; R 2 is NH 2 ; R 3 and R 4 are each hydrogen, halo, (C 1 -C 4 )alkyl optionally substituted with halo and the like; and X 1 to X 4 are each hydrogen, halo, hydroxy, (C 1 -C 4 )alkyl optionally substituted with halo and the like. These compounds have COX-2 inhibiting activity and thus useful for treating or preventing inflammation or other COX-2 related diseases.本发明涉及一种化合物的公式: 1 或其药用可接受的盐,其中A和R 1 各自为可选地取代的5至6成员的杂芳基,其中杂芳基可选择性熔合至碳环或5至6-杂芳基;R 2 为NH 2 ;R 3 和R 4 各自为氢,卤素,(C 1 -C 4 )烷基可选择性被卤素等取代;和X 1 至X 4 各自为氢,卤素,羟基,(C 1 -C 4 )烷基可选择性被卤素等取代。这些化合物具有COX-2抑制活性,因此可用于治疗或预防炎症或其它COX-2相关疾病。
-
[EN] LIVER X RECEPTOR (LXR) MODULATORS<br/>[FR] MODULATEURS DU RÉCEPTEUR X DU FOIE申请人:ALEXAR THERAPEUTICS INC公开号:WO2015035015A1公开(公告)日:2015-03-12Described herein are liver X receptor (LXR) modulators and methods of utilizing LXR modulators in the treatment of LXR-associated diseases, disorders or conditions. Also described herein are pharmaceutical compositions containing such compounds.本文描述了肝X受体(LXR)调节剂以及在治疗与LXR相关的疾病、疾病或症状中利用LXR调节剂的方法。本文还描述了含有这类化合物的药物组合物。
-
Synthesis of [11C]celecoxib: a potential PET probe for imaging COX-2 expression作者:Jaya Prabhakaran、Vattoly J. Majo、Norman R. Simpson、Ronald L. Van Heertum、J. John Mann、J. S. Dileep KumarDOI:10.1002/jlcr.1002日期:2005.10.30onamide or celecoxib (6) in 30% yield. However, under identical conditions, synthesis of [11C]celecoxib ([11C]6) was unsuccessful. Instead, trapping [11C]CH3I in an argon purged solution of catalytic amounts of Pd2(dba)3 and tri-o-tolylphosphine followed by the addition of the precursor 5 in DMF under argon and heating the mixture at 135°C for 4 min resulted in the incorporation of [11C]CH3 group.[11C] 塞来昔布(一种 COX-2 选择性抑制剂和治疗关节炎和疼痛的处方药)的标记已经实现。用于放射性标记的前体分子由 4-溴苯乙酮分 4 个步骤合成,总产率为 23%。N-[双-(4-甲氧基苯基)苯基甲基]-4-[5-(4-三丁基甲锡基苯基)-3-三氟甲基吡唑-1-基]苯磺酰胺(5)与碘甲烷在催化量的Pd2( dba)3、三邻甲苯基膦、CuCl 和过量的 K2CO3 在 DMF 中,然后用 20% 三氟乙酸对磺酰胺进行脱保护,得到 4-(5-p-tolyl-3-trifluoromethylpyrazol-1-yl) 苯磺酰胺或塞来昔布 (6 ) 的收益率为 30%。然而,在相同条件下,[11C]塞来昔布([11C]6)的合成未成功。反而,在氩气吹扫的催化量 Pd2(dba)3 和三邻甲苯基膦溶液中捕集 [11C]CH3I,然后在氩气下将前体 5 加入 DMF 中,并将混合物在 135°C
-
Structure of the products of condensation of hydroxylamine with trifluoromethyl-β-diketones: assignments of the diastereotopic protons of the 4-methylene group in 5-hydroxy-5-trifluoromethyl-Δ2-isoxazolines作者:Dionisia Sanz、Rosa M. Claramunt、Shiv P. Singh、Vinod Kumar、Ranjana Aggarwal、José Elguero、Ibon AlkortaDOI:10.1002/mrc.1676日期:2005.12The combined use of 1H NMR spectroscopy with theoretical calculations of chemical shifts (GIAO) and coupling constants (B3LYP/6‐311 ++G**) of a 5‐hydroxy‐5‐trifluoromethyl‐Δ2‐isoxazoline has enabled solving the problem of the assignments of the diastereotopic protons in this compound. This result has been extended to 5‐hydroxy‐5‐trifluoromethyl‐Δ2‐pyrazolines and the corresponding 5‐trichloromethyl
-
Highly Regioselective Synthesis of 1-Aryl-3,4,5-Substituted Pyrazoles作者:Francis Gosselin、Paul O’Shea、Robert Webster、Robert Reamer、Richard Tillyer、Edward GrabowskiDOI:10.1055/s-2006-956487日期:——A highly regioselective synthesis of 1-aryl-3,4,5-substituted pyrazoles based on the condensation of 1,3-diketones with arylhydrazines is described. The reaction proceeds at room temperature in N,N-dimethylacetamide and furnishes pyrazoles in 59-98% yields.
表征谱图
-
氢谱1HNMR
-
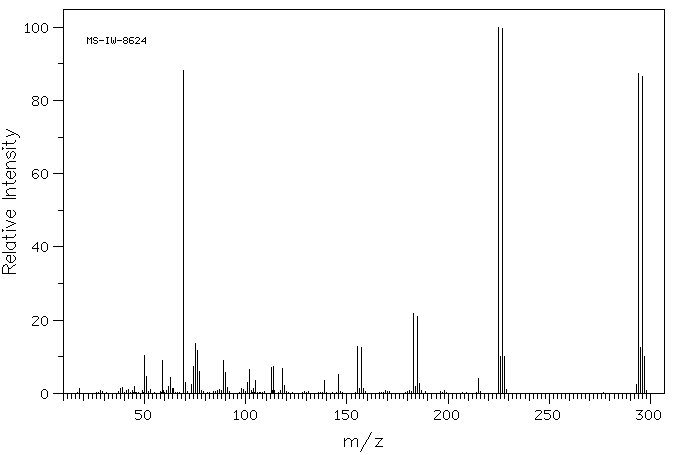
质谱MS
-
碳谱13CNMR
-
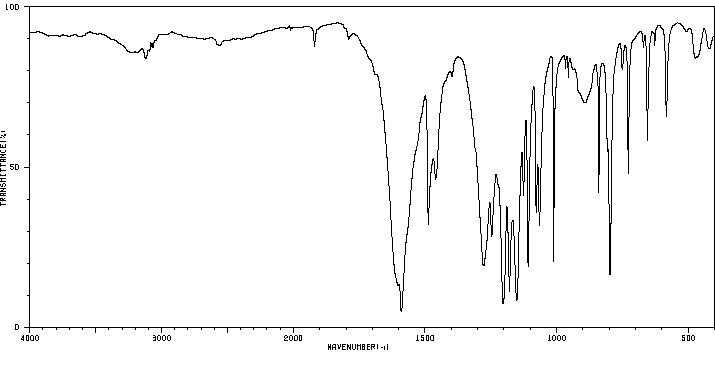
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷