4-(甲氨基)苯甲酸甲酯 | 18358-63-9
中文名称
4-(甲氨基)苯甲酸甲酯
中文别名
4-甲基氨基苯甲酸甲酯;4-(甲氨基)苯甲酸甲酯,98%
英文名称
methyl 4-(N-methyl)aminobenzoate
英文别名
methyl 4-(methylamino)benzoate
CAS
18358-63-9
化学式
C9H11NO2
mdl
MFCD00017198
分子量
165.192
InChiKey
LLAMGYUWYUMHCH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:94-96°C
-
沸点:277.5±23.0 °C(Predicted)
-
密度:1.125±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
稳定性/保质期:
常温常压下稳定,避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
安全说明:S26,S36/37/39,S45
-
危险品运输编号:1672
-
WGK Germany:3
-
海关编码:2922499990
-
危险品标志:F,C
-
危险类别码:R34,R11
-
RTECS号:NJ6700000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温下应密闭避光保存,并保持通风和干燥。
SDS
Version 1.0
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name METHYL 4-(METHYLAMINO)BENZOATE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
May cause sensitization by skin contact.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
METHYL 4-(METHYLAMINO)BENZOATE 18358-63-9 None None
Formula C9H11NO2
Molecular Weight 165,1900 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
ALDRICH www.molbase.com
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid breathing dust. Avoid
contact with eyes, skin, and clothing. Avoid prolonged or
repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling. Wash contaminated clothing before
reuse.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a dust mask type N95 (US) or type P1 (EN 143)
respirator.
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
Appearance Physical State: Solid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
ALDRICH www.molbase.com
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 1,825
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SENSITIZATION
Sensitization: Sensitizer.
Skin: May cause allergic skin reaction. The preceding data, or
interpretation of data, was determined using Quantitative
Structure Activity Relationship (QSAR) modeling.
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: May be harmful if inhaled. Material may be
irritating to mucous membranes and upper respiratory tract.
Ingestion: May be harmful if swallowed.
12 - Ecological Information
No data available.
13 - Disposal Considerations
ALDRICH www.molbase.com
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
INDICATION OF DANGER: Xi
Irritant.
R-PHRASES: 43
May cause sensitization by skin contact.
S-PHRASES: 36/37
Wear suitable protective clothing and gloves.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name METHYL 4-(METHYLAMINO)BENZOATE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
May cause sensitization by skin contact.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
METHYL 4-(METHYLAMINO)BENZOATE 18358-63-9 None None
Formula C9H11NO2
Molecular Weight 165,1900 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
ALDRICH www.molbase.com
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid breathing dust. Avoid
contact with eyes, skin, and clothing. Avoid prolonged or
repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling. Wash contaminated clothing before
reuse.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a dust mask type N95 (US) or type P1 (EN 143)
respirator.
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
Appearance Physical State: Solid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
ALDRICH www.molbase.com
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 1,825
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SENSITIZATION
Sensitization: Sensitizer.
Skin: May cause allergic skin reaction. The preceding data, or
interpretation of data, was determined using Quantitative
Structure Activity Relationship (QSAR) modeling.
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: May be harmful if inhaled. Material may be
irritating to mucous membranes and upper respiratory tract.
Ingestion: May be harmful if swallowed.
12 - Ecological Information
No data available.
13 - Disposal Considerations
ALDRICH www.molbase.com
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
INDICATION OF DANGER: Xi
Irritant.
R-PHRASES: 43
May cause sensitization by skin contact.
S-PHRASES: 36/37
Wear suitable protective clothing and gloves.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
4-甲氨基苯甲酸甲酯可作为有机中间体和医药中间体,广泛用于实验室研发及化工生产过程。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-二甲氨基苯甲酸甲酯 methyl 4-(N,N-dimethylamino)benzoate 1202-25-1 C10H13NO2 179.219 4-甲氨基苯甲酸 4-methylaminobenzoic acid 10541-83-0 C8H9NO2 151.165 4-氨基苯甲酸甲酯 4-methoxycarbonyl aniline 619-45-4 C8H9NO2 151.165 苯佐卡因 p-aminoethylbenzoate 94-09-7 C9H11NO2 165.192 N-甲基-N-亚硝基-4-氨基苯甲酸甲酯 methyl 4-(methyl(nitroso)amino)benzoate 18600-49-2 C9H10N2O3 194.19 对氨基苯甲酸 4-amino-benzoic acid 150-13-0 C7H7NO2 137.138 —— N-(2-carbomethoxyphenyl)-N-methylacetamide 37619-13-9 C11H13NO3 207.229 对硝基苯甲酸甲酯 4-nitrobenzoic acid methyl ester 619-50-1 C8H7NO4 181.148 —— methyl 4-[(tert-butoxycarbonyl)(methyl)amino]benzoate 741275-29-6 C14H19NO4 265.309 4-碘苯甲酸甲酯 methyl 4-iodobenzoate 619-44-3 C8H7IO2 262.047 对氯苯甲酸甲酯 Methyl 4-chlorobenzoate 1126-46-1 C8H7ClO2 170.595 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-二甲氨基苯甲酸甲酯 methyl 4-(N,N-dimethylamino)benzoate 1202-25-1 C10H13NO2 179.219 4-甲氨基苯甲酸 4-methylaminobenzoic acid 10541-83-0 C8H9NO2 151.165 —— methyl 4-((2-hydroxyethyl)(methyl)amino)benzoate —— C11H15NO3 209.245 —— methyl 4-((cyanomethyl)methylamino)benzoate 51934-23-7 C11H12N2O2 204.228 N-甲基-N-亚硝基-4-氨基苯甲酸甲酯 methyl 4-(methyl(nitroso)amino)benzoate 18600-49-2 C9H10N2O3 194.19 —— methyl 4-(methyl(phenyl)amino)benzoate —— C15H15NO2 241.29 —— methyl 4-(methyl(trifluoromethyl)amino)benzoate 34578-03-5 C10H10F3NO2 233.19 —— N-(4-methoxycarbonylphenyl)-N-methylcarbamoyl chloride 1235712-90-9 C10H10ClNO3 227.647 —— 4-[methyl(trimethylsilyl)amino]benzoic acid methyl ester 779355-83-8 C12H19NO2Si 237.374 [4-(甲基氨基)苯基]甲醇 (4-(methylamino)phenyl)methanol 181819-75-0 C8H11NO 137.181 4-甲基氨基苯甲醛 4-(methylamino)benzaldehyde 556-21-8 C8H9NO 135.166 —— methyl 4-[acryloyl(methyl)amino]benzoate 1081979-45-4 C12H13NO3 219.24 4-[苄基(甲基)氨基]苯甲酸 4-(benzyl-methyl-amino)-benzoic acid 25070-91-1 C15H15NO2 241.29 —— 4-[(methyl)(methylsulfonyl)amino]benzoic acid methyl ester 135036-55-4 C10H13NO4S 243.284 —— methyl 4-[(tert-butoxycarbonyl)(methyl)amino]benzoate 741275-29-6 C14H19NO4 265.309 —— Methyl 4-[methyl(2,2,3,3-tetrafluoropropyl)amino]benzoate —— C12H13F4NO2 279.23 —— methyl 4-(N-methylmethacrylamido)benzoate 839698-87-2 C13H15NO3 233.267 —— methyl 4-(methyl(3-oxo-3-phenylpropyl)amino)benzoate 89787-34-8 C18H19NO3 297.354 - 1
- 2
反应信息
-
作为反应物:描述:4-(甲氨基)苯甲酸甲酯 在 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 为溶剂, 以55%的产率得到[4-(甲基氨基)苯基]甲醇参考文献:名称:康维他汀A-4的NQO1选择性活化前药:合成和生物学评估。摘要:特定于肿瘤的前药治疗使抗肿瘤药的排他性传递降到最低。在这项工作中,我们报告了由活性药物CA-4,不同的自消灭性接头和NQO1反应性触发基团构成的四种NQO1可激活的康维他汀A-4前药的合成和生物学评估。体外抗增殖活性表明,前药4对过表达NQO1,紫杉醇抗性A549细胞,低氧暴露的A549和HepG2细胞的肿瘤细胞具有更大的选择性毒性,与康布雷他汀A-4相比,对正常细胞的损伤较小,前药1,2,和3。此外,基于机理研究,NQO1触发了前药4有效释放母体药物康普他汀A-4并杀死肿瘤细胞。此外,我们还证明,在体内条件下,前药4的抗癌作用和安全性均高于康培他汀A-4。因此,根据以上结果,NQO1可以用作释放抗癌剂的特定递送系统。此外,前药4可以作为开发特定抗癌药的候选药物。DOI:10.1016/j.bioorg.2020.104200
-
作为产物:描述:参考文献:名称:来自苄基叠氮化物的N-甲基苯胺摘要:在布朗斯台德酸或路易斯酸和Et 3 SiH的存在下,苄基叠氮化物可有效地转化为N-甲基苯胺。叠氮化物和4-正丁基苄基叠氮化物的组合似乎形成了氨基重氮三氯锡酸酯(II),该重氮经历重排成亚胺盐,然后被Et 3 SiH还原为N-甲基-4-正丁基苯胺。DOI:10.1016/s0040-4039(99)00148-3
文献信息
-
Di(aromatic) compounds and their use in human and veterinary medicine申请人:Centre International de Recherches Dermatologiques Galderma (Cird公开号:US05387594A1公开(公告)日:1995-02-07Di(aromatic) compounds corresponding to the following formula: ##STR1## in which: Ar represents either ##STR2## n=1 or 2 or: ##STR3## X represents a divalent radical, Z represents O, S or a divalent radical, and R.sub.1, R.sub.2, R.sub.3, R.sub.4 and R.sub.5 represent a hydrogen atom or various organic radicals, and the salts of the compounds of formula (I) when R.sub.1 is a carboxylic acid function. Use in human and veterinary medicine and in cosmetics.对应以下公式的二芳基化合物:##STR1## 其中:Ar代表##STR2## n=1或2或:##STR3## X代表二价基团,Z代表O、S或二价基团,R.sub.1、R.sub.2、R.sub.3、R.sub.4和R.sub.5代表氢原子或各种有机基团,以及当R.sub.1是羧酸功能时,公式(I)化合物的盐。用于人类和兽医学以及化妆品。
-
Preparation of Unsymmetrical Sulfonylureas from <i>N</i>,<i>N</i>‘-Sulfuryldiimidazoles作者:Serge Beaudoin、Kenneth E. Kinsey、James F. BurnsDOI:10.1021/jo026505k日期:2003.1.1use in parallel synthesis. We have developed a method for preparing sterically congested sulfonylureas based on N,N'-sulfuryldiimidazole that is both convenient and amenable to parallel synthesis. Sequential activation by way of alkylation of the imidazole group using methyl triflate followed by nucleophilic displacement with a variety of amines and anilines afford the unsymmetrical sulfonylurea. Sulfonylureas
-
Pyrrolidine derivatives申请人:——公开号:US20020049243A1公开(公告)日:2002-04-25The present invention relates to pyrrolidine derivatives and dimeric forms and/or pharmaceutically acceptable esters, and/or salts thereof. The compounds are useful as inhibitors of metalloproteases, e.g. zinc proteases, particularly zinc hydrolases, and which are effective in treating disease states are associated with vasoconstriction of increasing occurrences.
-
Matrix metalloproteinase inhibitors申请人:——公开号:US20030078276A1公开(公告)日:2003-04-24Compounds are provided that bind allosterically to the catalytic domain of MMP-13 and comprise a hydrophobic group, first and second hydrogen bond acceptors and at least one, and preferably both, of a third hydrogen bond acceptor and a second hydrophobic group. Cartesian coordinates for centroids of the above features are defined in the specification. When the ligand binds to MMP-13, the first, second and third (when present) hydrogen bond acceptors bond respectively with Thr245, Thr 247 and Met 253, the first hydrophobic group locates within the S1′ channel of MMP-13 and the second hydrophobic group (when present) is relatively open to solvent. The compounds specifically inhibit the matrix metalloproteinase-13 enzyme and thus are useful for treating diseases resulting from tissue breakdown, such as heart disease, multiple sclerosis, arthritis, atherosclerosis, and osteoporosis.
-
Cu-Catalyzed Sequential Dehydrogenation–Conjugate Addition for β-Functionalization of Saturated Ketones: Scope and Mechanism作者:Xiaoming Jie、Yaping Shang、Xiaofeng Zhang、Weiping SuDOI:10.1021/jacs.6b01337日期:2016.5.4The first copper-catalyzed direct β-functionalization of saturated ketones is reported. This protocol enables diverse ketones to couple with a wide range of nitrogen, oxygen and carbon nucleophiles in generally good yields under operationally simple conditions. The detailed mechanistic studies including kinetic studies, KIE measurements, identification of reaction intermediates, EPR and UV-visible报道了第一个铜催化的饱和酮直接β-官能化。该协议使不同的酮能够在操作简单的条件下以通常良好的产率与广泛的氮、氧和碳亲核试剂结合。进行了详细的机理研究,包括动力学研究、KIE 测量、反应中间体的鉴定、EPR 和紫外可见实验,结果表明该反应是通过一种新型的基于自由基的脱氢生成烯酮和随后的共轭加成序列进行的。
表征谱图
-
氢谱1HNMR
-
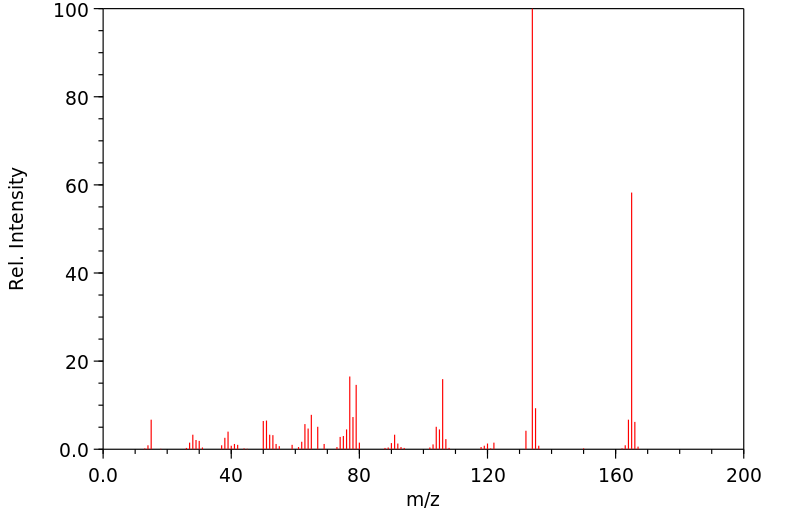
质谱MS
-
碳谱13CNMR
-
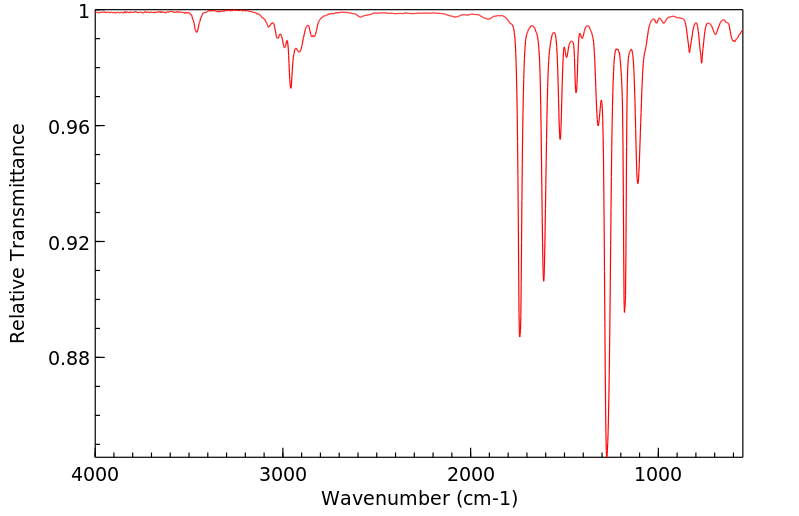
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫