1,3-二羟基丙酮 | 96-26-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75-80 °C
-
沸点:107.25°C (rough estimate)
-
密度:1.1385 (rough estimate)
-
溶解度:溶于 DMSO(>112.4 mg/mL);乙醇溶液(>5.09 mg/mL)
-
LogP:-1.95 at 20℃
-
表面张力:68.85mN/m at 1g/L and 20℃
-
物理描述:Solid
-
颜色/状态:Crystalline powder
-
气味:Characteristic odor
-
味道:Sweet, cooling taste
-
蒸汽压力:4.35X10-5 mm Hg at 25 °C
-
分解:When heated to decomposition it emits acrid smoke and irritating vapors.
-
保留指数:2068;2075
-
稳定性/保质期:
稳定、易燃、吸湿性强。
计算性质
-
辛醇/水分配系数(LogP):-1.4
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
ADMET
安全信息
-
安全说明:S24/25
-
海关编码:2914400090
-
危险品运输编号:OTH
-
WGK Germany:1
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:Refrigerator (+4℃)
SDS
制备方法与用途
二羟基丙酮是一种天然存在的酮糖,具有生物可降解性、可食用且对人体和环境无毒害。作为多功能添加剂,它广泛应用于化妆品、医药和食品行业。
二羟基丙酮二羟基丙酮(Dihydroxyacetone, DHA)的化学名为1,3-二羟基-2-丙酮,是一种常用的化妆品成分,具有皮肤保湿、仿晒以及染发的功能。截至当前,DHA不属欧盟化妆品监管条例(Cosmetic Regulation (EC) No. 1223/2009)的监管范围。
2008年,欧盟消费者安全科学委员会(Scientific Committee on Consumer Safety, SCCS)收到一份企业提交的资料,用以支持DHA作为化妆品成分的安全性。研究数据表明,在化妆品中使用浓度不超过10%的DHA作为仿晒剂是安全的;此外,喷雾类化妆品中的DHA浓度若不超过14%,也是安全的。
2019年5月,SCCS再次收到申请,评估在驻留类(非氧化型)染发化妆品中使用浓度不超过6.25% DHA的安全性。根据企业提供的毒代动力学、皮肤刺激性和致敏性、急性毒性、生殖毒性、致突变及致癌等安全性评价数据,欧盟消费者安全科学委员会得出以下结论:在驻留类(非氧化型)染发化妆品中使用浓度不超过6.25%的DHA是安全的;与美黑乳液或浓度不超过10% DHA的面霜一同使用时亦被认为是安全的。
性状二羟基丙酮为带有甜味的白色粉末状结晶。
应用 化妆品工业-
作为防晒霜原料:二羟基丙酮主要用作化妆品配方中的原料,特别适用于防晒霜。它能防止皮肤水分过度蒸发,起到保湿、防晒和防紫外线辐射的作用。
-
模拟日晒肤色:DHA中的酮官能团可与皮肤角蛋白的氨基酸和氨基基团发生反应形成褐色聚合物,使皮肤产生类似长时间暴露于阳光下的自然棕色或棕褐色。因此,它可以作为日晒肤色的模拟剂。
二羟基丙酮是糖代谢过程中的中间产物,在糖代谢过程中起着重要作用,具有降低脂肪、提高瘦肉率的效果。
用途上下游信息
反应信息
-
作为反应物:参考文献:名称:用于酶催化质子转移、氢化物转移和脱羧的活化氧双阴离子结合域:特异性和酶结构摘要:为催化其各自的磷酸二价阴离子截断底物的反应,报告了以下氧二阴离子的用于激活酵母丙糖磷酸异构酶 (ScTIM)、酵母乳清酸单磷酸脱羧酶 (ScOMPDC) 和人肝甘油 3-磷酸脱氢酶 (hlGPDH) 的动力学参数:HPO32 –、FPO32–、S2O32–、SO42– 和 HOPO32–。Oxydianions 与这些未配体的酶弱结合,并与过渡态复合物 (E·S‡) 紧密结合,固有的氧二价阴离子 Gibbs 结合自由能范围为 -8.4 kcal/mol,用于激活 hlGPDH 催化的 FPO32 还原乙醇醛 - 3.0 kcal/mol 用于通过 HOPO32– 激活 ScOMPDC 催化的 1-β-d-赤呋喃糖基)乳清酸的脱羧。观察到不同氧二阴离子结合域的特异性的微小差异。我们提出,与 ScTIM 和 ScOMPDC 的激活相比,FPO32– 和 S2O32– 分别激活 hlGPDH 的大DOI:10.1021/ja5123842
-
作为产物:参考文献:名称:Seeing Mountains in Mole Hills: Geographical-Slant Perception摘要:当观察者直接面向山坡的倾斜时,他们对山坡的倾斜意识被极大地高估,但运动估计要准确得多。本研究探讨了在观察者被允许看到山坡侧面时是否会出现类似的结果。观察者在真实(实验1)和虚拟(实验2)环境中观察山坡的横截面,并通过口头估计、调整圆盘的横截面以及用看不见的手调整板以匹配倾斜度来进行估计。我们发现,当观察者直接面对山坡时的结果与观察横截面时的结果相似。即使从侧面观察时山坡的角度是直接明显的,但倾斜感知仍然被严重高估。DOI:10.1111/1467-9280.00377
-
作为试剂:描述:5-(1-氮杂环丙基)-2,4-二硝基苯甲酰胺 在 potassium carbonate 、 1,3-二羟基丙酮 作用下, 以 甲醇 为溶剂, 反应 0.25h, 生成 5-(氮丙啶-1-基)-4-羟基氨基-2-硝基苯甲酰胺 、 5-(aziridin-1-yl)-2-hydroxylamino-4-nitrobenzamide参考文献:名称:WO2007/74344摘要:公开号:
文献信息
-
[EN] LYMPHATIC SYSTEM-DIRECTING LIPID PRODRUGS<br/>[FR] PROMÉDICAMENTS LIPIDIQUES ORIENTANT VERS LE SYSTÈME LYMPHATIQUE申请人:ARIYA THERAPEUTICS INC公开号:WO2019046491A1公开(公告)日:2019-03-07The present invention provides lymphatic system-directing lipid prodrugs, pharmaceutical compositions thereof, methods of producing such prodrugs and compositions, as well as methods of improving the bioavailability or other properties of a therapeutic agent that comprises part of the lipid prodrug. The present invention also provides methods of treating a disease, disorder, or condition such as those disclosed herein, comprising administering to a patient in need thereof a provided lipid prodrug or a pharmaceutical composition thereof.本发明提供了淋巴系统定向脂质前药,其制药组合物,制备这种前药和组合物的方法,以及改善作为脂质前药一部分的治疗剂的生物利用度或其他性质的方法。本发明还提供了治疗疾病、紊乱或症状的方法,包括向需要的患者施用所提供的脂质前药或其制药组合物。
-
[EN] LIPID PRODRUGS OF JAK INHIBITORS AND USES THEREOF<br/>[FR] PROMÉDICAMENTS LIPIDIQUES D'INHIBITEURS DE JAK ET LEURS UTILISATIONS申请人:PURETECH LYT INC公开号:WO2020176859A1公开(公告)日:2020-09-03The present invention provides lymphatic system-directing lipid prodrugs, pharmaceutical compositions thereof, methods of producing such prodrugs and compositions, and methods of improving the bioavailability or other properties of a therapeutic agent that comprises part of the lipid prodrug. The present invention also provides methods of treating a disease, disorder, or condition such as those disclosed herein, comprising administering to a patient in need thereof a disclosed lipid prodrug or a pharmaceutical composition thereof.本发明提供了淋巴系统定向脂质前药,其药物组成物,生产这种前药和组成物的方法,以及改善作为脂质前药一部分的治疗剂的生物利用度或其他性质的方法。本发明还提供了治疗疾病、紊乱或状况的方法,例如本文所披露的那些,包括向需要的患者施用所披露的脂质前药或其药物组成物。
-
[EN] CLEAVABLE MULTI-ALCOHOL-BASED MICROCAPSULES<br/>[FR] MICROCAPSULES CLIVABLES À BASE D'ALCOOLS MULTIPLES申请人:FIRMENICH & CIE公开号:WO2021023645A1公开(公告)日:2021-02-11The present invention relates to a new process for the preparation of microcapsules based on cleavable multi-alcohols. Cleavable multi-alcohol-based microcapsules are also an object of the invention. Perfuming compositions and consumer products comprising said capsules, in particular perfumed consumer products in the form of home care or personal care products, are also part of the invention.
-
BENZAZEPINE DERIVATIVE, PROCESS FOR PRODUCING THE SAME, AND USE申请人:Takeda Chemical Industries, Ltd.公开号:EP1422228A1公开(公告)日:2004-05-26The present invention provides a novel benzazepine derivative represented by formula : wherein, R1 is a 5- or 6-membered aromatic ring, R2 is lower alkyl group, etc., Y is an optionally substituted imino group, ring A and ring B are independently an optionally substituted aromatic ring, W is formula -W1-X2-W2- (W1 and W2 are independently S(O)m1 (m1 is 0, 1 or 2), etc., and X2 is an optionally substituted alkylene groupetc. ), a preparation method and use thereof.
-
Synthesis and Liver Microsomal Metabolic Stability Studies of a Fluorine‐Substituted δ‐Tocotrienol Derivative作者:Xingui Liu、Saikat Poddar、Lin Song、Howard Hendrickson、Xuan Zhang、Yaxia Yuan、Daohong Zhou、Guangrong ZhengDOI:10.1002/cmdc.201900676日期:2020.3.18A fluoro-substituted δ-tocotrienol derivative, DT3-F2, was synthesized. This compound was designed to stabilize the metabolically labile terminal methyl groups of δ-tocotrienol by replacing one C-H bond on each of the two methyl groups with a C-F bond. However, in vitro metabolic stability studies using mouse liver microsomes revealed an unexpected rapid enzymatic C-F bond hydrolysis of DT3-F2. To
表征谱图
-
氢谱1HNMR
-
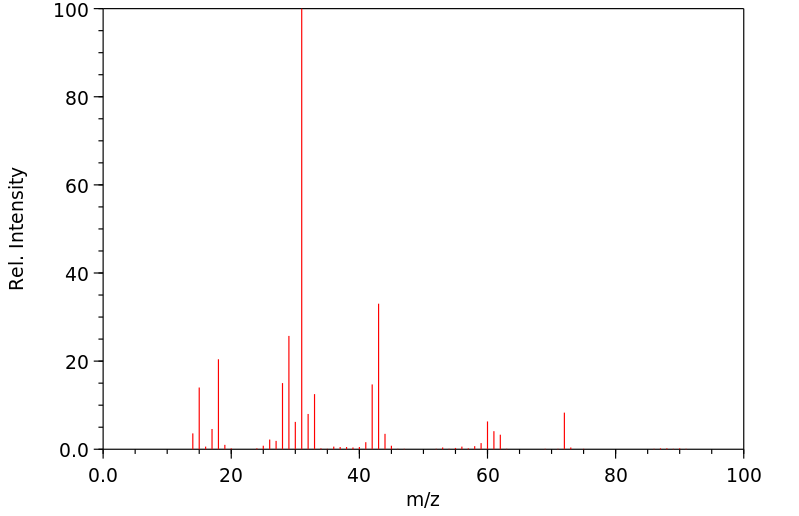
质谱MS
-
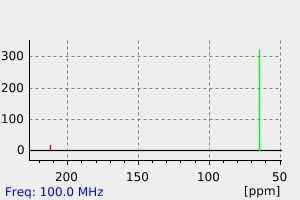
碳谱13CNMR
-
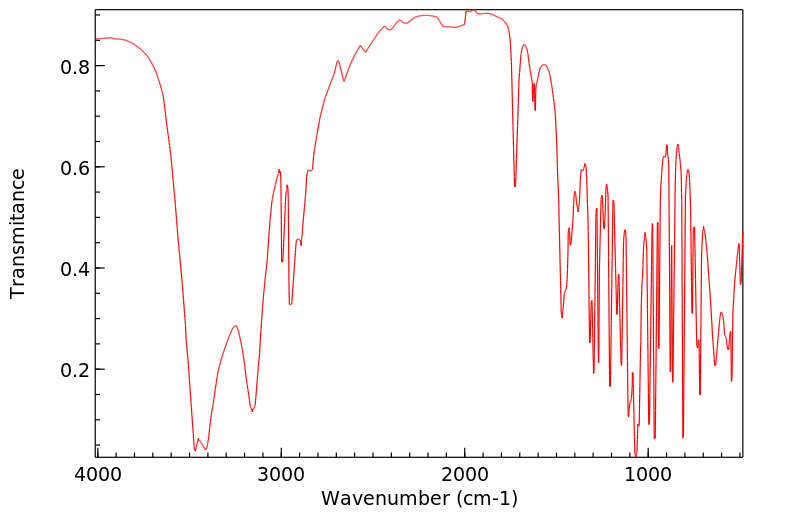
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息