2-溴萘 | 580-13-2
中文名称
2-溴萘
中文别名
Β-溴代萘;2-溴化萘;2-溴代萘;β-溴萘;β-溴代萘
英文名称
2-bromonaphthalene
英文别名
2-bromonaphtalene;2-bromonapthalene;2-naphthyl bromide;β-bromo-naphthalene
CAS
580-13-2
化学式
C10H7Br
mdl
MFCD00004051
分子量
207.07
InChiKey
APSMUYYLXZULMS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:52-55 °C (lit.)
-
沸点:281-282 °C (lit.)
-
密度:1,605 g/cm3
-
闪点:>230 °F
-
溶解度:溶于甲醇:50mg/mL,澄清,无色至黄色
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
WGK Germany:3
-
海关编码:29036990
-
安全说明:S26,S36,S36/37,S45
-
危险品运输编号:UN 1230 3/PG 2
-
危险品标志:Xn
-
危险类别码:R22,R36
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:本品应密封避光保存。
SDS
| Name: | 2-Bromonaphtalene 99% (GC) Material Safety Data Sheet |
| Synonym: | Tert-Butyl Bromid |
| CAS: | 580-13-2 |
Synonym:Tert-Butyl Bromid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 580-13-2 | 2-Bromonaphthalene | 99 (GC) | 209-452-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 580-13-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 281 - 282 deg C
Freezing/Melting Point: 52 - 55 deg C
Autoignition Temperature: Not available.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water: slightly soluble in water
Specific Gravity/Density:
Molecular Formula: C10H7Br
Molecular Weight: 207.07
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases, bromine fumes, bromine.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 580-13-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Bromonaphthalene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 580-13-2: No information available.
Canada
CAS# 580-13-2 is listed on Canada's NDSL List.
CAS# 580-13-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 580-13-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,6-二溴萘 1,6-dibromonaphthalene 19125-84-9 C10H6Br2 285.966 1-溴代萘 1-Bromonaphthalene 90-11-9 C10H7Br 207.07 1,3-二溴萘 1,3-dibromonaphthalene 52358-73-3 C10H6Br2 285.966 1,2-二溴萘 1,2-dibromonaphthalene 5438-13-1 C10H6Br2 285.966 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-溴代萘 1-Bromonaphthalene 90-11-9 C10H7Br 207.07 1,7-二溴萘 1,7‐dibromonaphthalene 58258-65-4 C10H6Br2 285.966 —— 1,4,6-tribromonaphthalene 72647-08-6 C10H5Br3 364.862 2-溴-6-碘萘 2-bromo-6-iodonaphthalene 389806-32-0 C10H6BrI 332.966 7-溴-1-羟基萘 7-bromonaphthalen-1-ol 91270-69-8 C10H7BrO 223.069 —— 2-bromo-6-ethylnaphthalene 67668-21-7 C12H11Br 235.123 3-溴-1-羟基萘 3-bromo-1-naphthol 90767-17-2 C10H7BrO 223.069 2-溴-1-萘酚 2-bromo-1-naphthol 771-15-3 C10H7BrO 223.069 —— 6-Bromo-[2,2';6',2'']ternaphthalene 817210-33-6 C30H19Br 459.4
反应信息
-
作为反应物:参考文献:名称:炔烃、氯化芳基锌和碘甲烷的铑催化三组分反应产生有/无 1,4-迁移的三取代/四取代烯烃摘要:发现在铑催化剂存在下进行炔烃、芳基氯化锌和碘甲烷的三组分反应,以产生高产率的三取代/四取代烯烃。通常的芳基氯化锌仅产生三取代的烯烃,通过迁移的碳化 - 交叉偶联序列产生,其中 1,4-Rh 从烯基碳迁移到芳基碳。相比之下,5 元杂芳基氯化锌仅通过碳化-交叉偶联途径产生四取代烯烃,而没有 1,4-迁移。DOI:10.1021/acs.orglett.2c02299
-
作为产物:参考文献:名称:使用三溴化四丁基铵或三碘化铯对有机三氟硼酸盐进行卤代硼烷化摘要:报道了在水介质中使用市售的三溴化四丁基铵(TBATB)或三碘化铯对有机三氟硼酸盐进行卤代硼烷化。事实证明,这种温和的不含过渡金属的方法可耐受多种官能团。观察到高的区域选择性和化学选择性。两个合成路线(ż)从炔-dibromoalkenes,通过stereodefined(ż)-2- bromoalkenyltrifluoroborates和(Ž)-1,2-双(硼烷基)alkenyltrifluoroborates,已经使用TBATB介导bromodeboronation作为关键步骤开发的。DOI:10.1016/j.tet.2012.03.016
-
作为试剂:描述:7-氮杂吲哚 在 2-溴萘 、 potassium carbonate 作用下, 以 DMF (N,N-dimethyl-formamide) 、 氩 为溶剂, 反应 24.0h, 以44%的产率得到1-(naphthalen-2-yl)-1H-indole参考文献:名称:Substituted pyrroline kinase inhibitors摘要:本发明涉及新型取代吡咯烯化合物,可用作激酶抑制剂,并用于治疗或改善激酶介导的疾病的方法。公开号:US20040006095A1
文献信息
-
Pd-catalyzed carbonylative access to aroyl phosphonates from (hetero)aryl bromides
-
Synthese und flüssigkristalline Eigenschaften 2,6-disubstituierter Naphthaline
-
Substituted imidazol-pyridazine derivatives申请人:——公开号:US20030229096A1公开(公告)日:2003-12-11The present invention relates to compounds of formula 1 wherein A is an unsubstituted or substituted cyclic group; and R is hydrogen or lower alkyl; or a pharmaceutically acceptable acid addition salt thereof. These compounds are NMDA NR-2B receptor subtype specific blockers and are useful in the treatment of neurodegeneration, depression and pain.本发明涉及以下式的化合物 1 其中A是未取代或取代的环状基团;以及 R是氢或较低的烷基; 或其药学上可接受的酸盐。这些化合物是NMDA NR-2B受体亚型特异性阻断剂,对于治疗神经退行性疾病、抑郁症和疼痛具有用处。
-
Fungicides for the control of take-all disease of plants申请人:Monsanto Company公开号:US05498630A1公开(公告)日:1996-03-12A method of controlling Take-All disease of plants by applying a fungicide of the formula ##STR1## wherein Z1 and Z2 are C and are part of an aromatic ring which is benzothiophene; and A is selected from --C(X)-amine wherein the amine is an unsubstituted, monosubstituted or disubstituted amino radical, --C(O)--SR.sub.3, --NH--C(X)R.sub.4, and --C(.dbd.NR.sub.3)--XR.sub.7 ; B is --W.sub.m --Q(R.sub.2).sub.3 or selected from O-tolyl, 1-naphthyl, 2-naphthyl, and 9-phenanthryl, each optionally substituted with halogen or R.sub.4 ; Q is C, Si, Ge, or Sn; W is --C(R.sub.3).sub.p H.sub.(2-p) --; or when Q is C, W is selected from --C(R.sub.3).sub.p H(.sub.2-p), --N(R.sub.3).sub.m H(.sub.1-m)--, --S(O)p--, and --O--; X is 0 or S; n is 0, 1, 2, or 3; m is 0 or 1; p is 0, 1, or 2; each R and R.sub.2 is independently defined herein; R.sub.3 is C.sub.1 -C.sub.4 alkyl; R.sub.4 is C.sub.1 -C.sub.4 alkyl, haloalkyl, alkoxy, alkylthio, alkylamino, or dialkylamino; and R.sub.7 is C.sub.1 -C.sub.4 alkyl, haloalkyl, or phenyl, optionally substituted with halo, nitro, or R.sub.4 ; or an agronomic salt thereof.一种通过施用公式##STR1##的杀菌剂来控制植物全蚀病的方法,其中Z1和Z2为C,并且是苯并噻吩的芳香环的一部分;A从--C(X)-胺中选择,其中胺是未取代的、单取代的或双取代的氨基基团,--C(O)--SR.sub.3,--NH--C(X)R.sub.4和--C(.dbd.NR.sub.3)--XR.sub.7;B为--W.sub.m --Q(R.sub.2).sub.3或从O-甲苯基、1-萘基、2-萘基和9-菲基中选择,每个都可以选择性地用卤素或R.sub.4取代;Q为C、Si、Ge或Sn;W为--C(R.sub.3).sub.p H.sub.(2-p)--;或当Q为C时,W从--C(R.sub.3).sub.p H(.sub.2-p),--N(R.sub.3).sub.m H(.sub.1-m)--,--S(O)p--和--O--中选择;X为0或S;n为0、1、2或3;m为0或1;p为0、1或2;每个R和R.sub.2在此独立定义;R.sub.3为C.sub.1 -C.sub.4烷基;R.sub.4为C.sub.1 -C.sub.4烷基、卤代烷基、烷氧基、烷硫基、烷基氨基或二烷基氨基;R.sub.7为C.sub.1 -C.sub.4烷基、卤代烷基或苯基,可以选择性地用卤素、硝基或R.sub.4取代;或其农艺学盐。
-
Highly Diastereoselective Crown Ether Catalyzed Arylogous Michael Reaction of 3-Aryl Phthalides作者:Marina Sicignano、Antonella Dentoni Litta、Rosaria Schettini、Francesco De Riccardis、Giovanni Pierri、Consiglia Tedesco、Irene Izzo、Giorgio Della SalaDOI:10.1021/acs.orglett.7b02113日期:2017.8.18The first arylogous Michael reaction of 3-aryl phthalides has been developed. The reaction, promoted by catalytic amounts of KOH or K3PO4 and dibenzo-18-crown-6, affords the corresponding 3,3-disubstituted phthalides in good to high yields and as single diastereomers in nearly all studied cases.
表征谱图
-
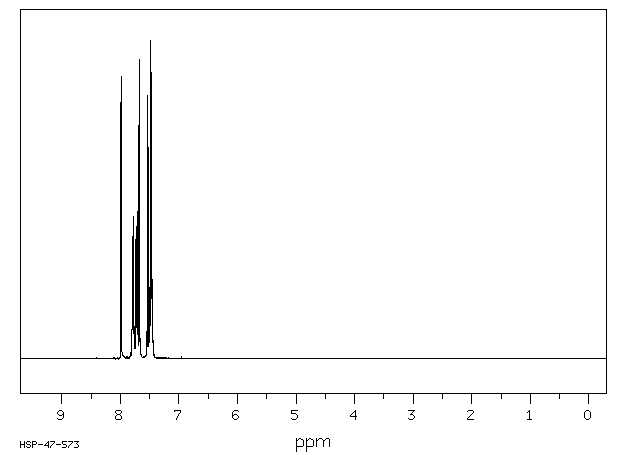
氢谱1HNMR
-
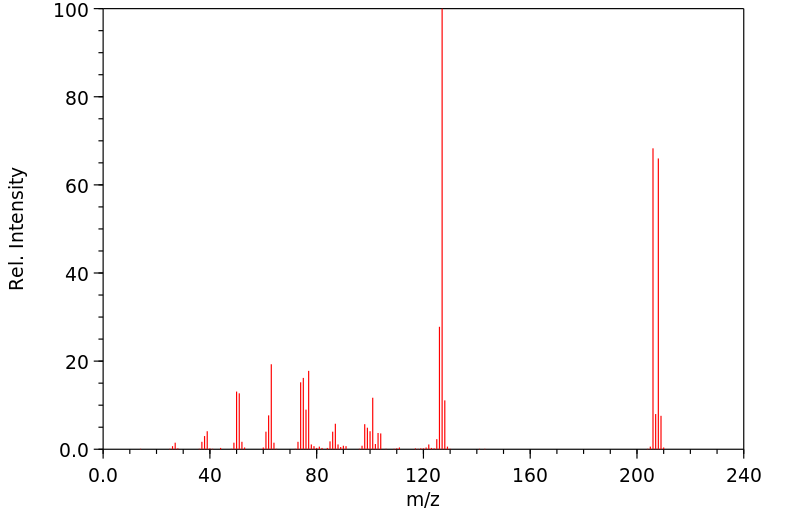
质谱MS
-
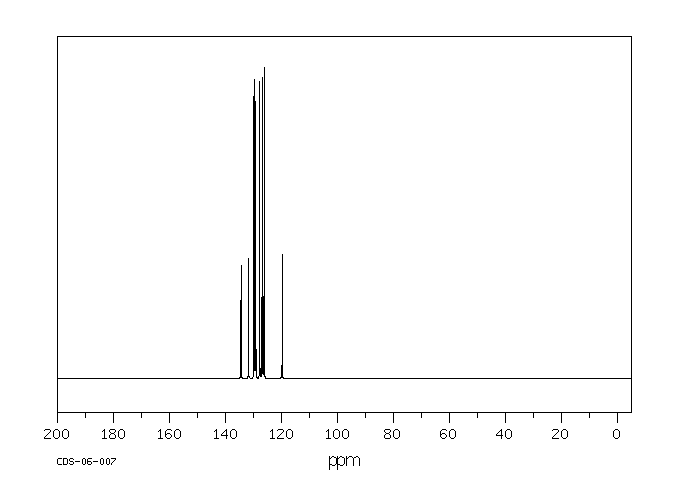
碳谱13CNMR
-
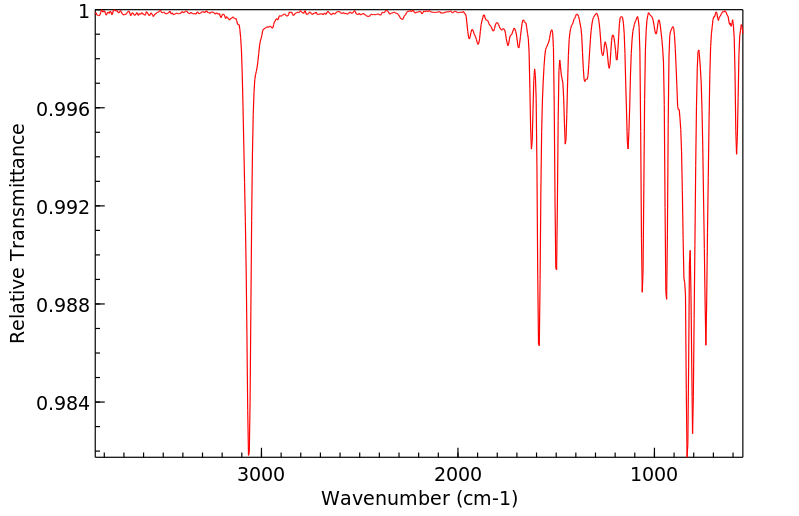
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮