2-溴(正)壬烷 | 2216-35-5
中文名称
2-溴(正)壬烷
中文别名
2-溴壬烷;2-溴正壬烷
英文名称
2-bromononane
英文别名
——
CAS
2216-35-5
化学式
C9H19Br
mdl
——
分子量
207.154
InChiKey
JQEFZTLHNWFZDD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:208-209°C
-
密度:1,081 g/cm3
-
闪点:208-209°C
-
稳定性/保质期:
避免让氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:10
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
海关编码:2903399090
-
储存条件:请将容器密封后,存放在干燥、阴凉的地方,并使用紧密的容器储存。
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:温和条件下有机卤化物的光诱导羟基化。摘要:本文介绍的是有机卤化物的光诱导羟基化作用,使人们可以轻而易举地接触到一系列官能化的酚和脂肪醇。这些反应通常在温和的反应条件下进行,不需要光催化剂或强碱,并且显示出较宽的底物范围以及优异的官能团耐受性。这项工作突显了NaI的独特作用,它可以在温和的反应条件下进行具有挑战性的转化。DOI:10.1021/acs.orglett.9b03317
-
作为产物:参考文献:名称:van Gysegem, Chemisches Zentralblatt, 1907, vol. 78, # I, p. 530摘要:DOI:
文献信息
-
Synthesis, Properties, and Crystal Structure of Silyl Nitronates (Silyl Esters ofaci-Nitroalkanes): Towards theSN2 Reaction Path with Retention of Configuration at Silicon作者:Ernest W. Colvin、Albert K. Beck、Bahram Bastani、Dieter Seebach、Yasushi Kai、Jack D. DunitzDOI:10.1002/hlca.19800630320日期:1980.4.23the preparation of silyl nitronates is described (see 1–10). NMR. spectral investigations indicate a rapid 1,3-silyl migration process, with an activation energy of about 10 kcal mol−1. X-ray crystallographic studies on the silyl nitronates 3 and 8 show structures that lean towards an SN2 retention pathway at silicon.
-
Photoinduced, Copper-Catalyzed Alkylation of Amides with Unactivated Secondary Alkyl Halides at Room Temperature作者:Hien-Quang Do、Shoshana Bachman、Alex C. Bissember、Jonas C. Peters、Gregory C. FuDOI:10.1021/ja4126609日期:2014.2.5The development of a mild and general method for the alkylation of amides with relatively unreactive alkyl halides (i.e., poor substrates for SN2 reactions) is an ongoing challenge in organic synthesis. We describe herein a versatile transition-metal-catalyzed approach: in particular, a photoinduced, copper-catalyzed monoalkylation of primary amides. A broad array of alkyl and aryl amides (as well as
-
The Fast and Selective Reduction of Organic Halides by Lithium Hydrotri-<i>s</i>-butylborate作者:Sunggak Kim、Kyu Yang YiDOI:10.1246/bcsj.58.789日期:1985.2Lithium hydrotri-s-butylborate is shown to be a powerful reducing agent in its reducing abilitie: toward organic halides and discriminates structurally different organic halides due to the bulkiness of the reagent Essentially complete utilization of the hydride of the reagent for the reduction of organic halides is also of synthetic significance.
-
Mercury-assisted solvolyses of alkyl halides作者:A. McKillop、M.E. FordDOI:10.1016/s0040-4020(01)97118-2日期:1974.1wide variety of alkyl halides with mercury(I) and/or (II) nitrate in 1,2-dimethoxyethane, mercury(II) acetate in acetic acid, aqueous mercury(II) perchlorate, and mercury(II) perchlorate in alcohol solvents have been investigated; as a result, simple high yield procedures for the conversion of alkyl halides into the corresponding nitrate esters, acetate esters, alcohols and ethers have been developed.
-
Copper nanoparticle-catalyzed cross-coupling of alkyl halides with Grignard reagents作者:Ju Hyun Kim、Young Keun ChungDOI:10.1039/c3cc46419a日期:——A cross-coupling reaction between alkyl bromides and chlorides and various Grignard reagents was carried out in the presence of commercially available copper or copper oxide nanoparticles as a catalyst and an alkyne additive. The catalytic system shows high activity, a broad scope, and good functional group tolerance.
表征谱图
-
氢谱1HNMR
-
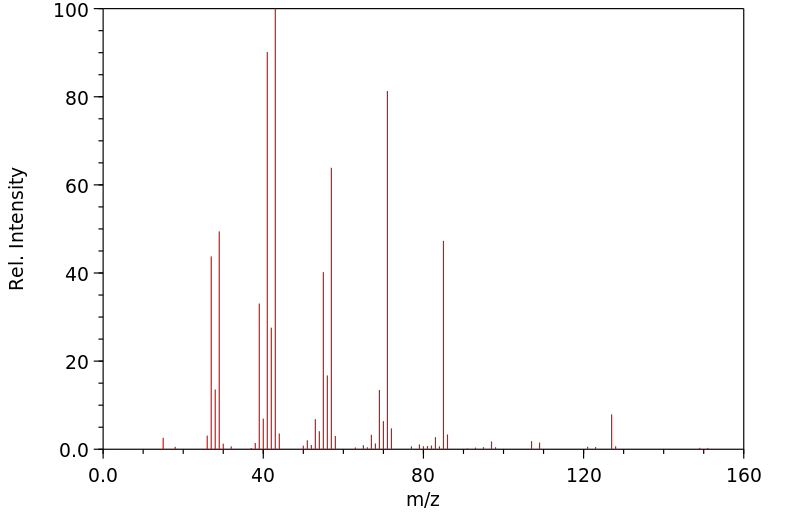
质谱MS
-
碳谱13CNMR
-
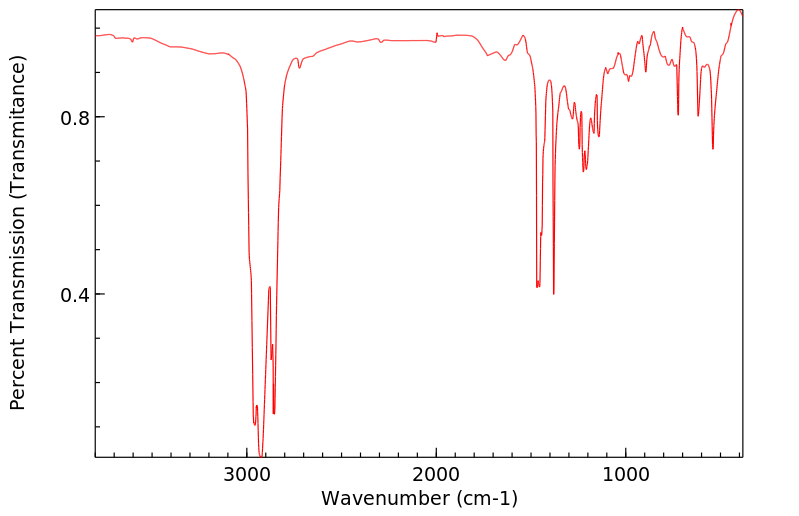
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4