1-丙基环戊烯 | 3074-61-1
中文名称
1-丙基环戊烯
中文别名
——
英文名称
1-propylcyclopentene
英文别名
1-n-propylcyclopentene;n-propylcyclopentene;1-propyl-cyclopentene;1-Propyl-cyclopenten;1-Propyl-cyclopenten-(1);1-Propyl-cyclopent-1-en
CAS
3074-61-1
化学式
C8H14
mdl
——
分子量
110.199
InChiKey
FLWGCAJANMGQBB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-100.3°C
-
沸点:132.05°C
-
密度:0.7978
-
保留指数:836;838;839;841;840;831;838;841;862;868;836.5;839.2;836;839;839;836
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:8
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:参考文献:名称:Kawaguchi, Mamoru; Hayashi, Osamu; Sakai, Noriyuki, Agricultural and Biological Chemistry, 1986, vol. 50, # 12, p. 3107 - 3112摘要:DOI:
-
作为产物:参考文献:名称:Plate; Melnikow, Zhurnal Obshchei Khimii, 1959, vol. 29, p. 1064,1068摘要:DOI:
文献信息
-
Aliphatic Friedel-Crafts reactions. Part VIII. Preparation of unsaturated ketones by the acylation of 1-alkylcyclopentenes作者:J. K. Groves、N. JonesDOI:10.1039/j39690000608日期:——Reaction of 1-ethylcyclopentene with zinc chloride–acetic anhydride affords 5-acetyl-1-ethylcyclopentene (39%) and 1-acetyl-2-ethylidenecyclopentane (13%). Similar products are obtained in the acetylation of other n-alkylcyclopentenes, with the exception of 1-methylcyclopentene which yields a mixture of 5-acetyl-1-methylcyclopentene and 1-acetyl-2-methylcyclopentene in a ratio of ca. 3 : 2. Treatment
-
Convenient ‘one-flask’ synthesis of olefins. Reaction of α-chloroketones with Grignard reagents and lithium作者:José Barluenga、Miguel Yus、Pablo BernadDOI:10.1039/c3978000847a日期:——Olefins and diolefins with the double bonds in predetermined positions are prepared in a one-step process in moderate to good yields by the reaction of α-chloroketones with Grignard reagents and then with lithium.
-
B(C <sub>6</sub> F <sub>5</sub> ) <sub>3</sub> ‐Catalyzed <i>E</i> ‐Selective Isomerization of Alkenes作者:Betty A. Kustiana、Salma A. Elsherbeni、Thomas G. Linford‐Wood、Rebecca L. Melen、Matthew N. Grayson、Louis C. MorrillDOI:10.1002/chem.202202454日期:2022.11.11report the B(C6F5)3-catalyzed E-selective isomerization of alkenes. The transition-metal-free method is applicable across a diverse array of readily accessible substrates, accessing a broad range of synthetically useful products containing versatile stereodefined internal alkenes. Synthetic and computational mechanistic studies indicate that the isomerization proceeds along competing 1,2-hydride shift
-
Barluenga, Jose; Yus, Miguel; Concellon, Jose M., Journal of Chemical Research, Miniprint, 1980, # 2, p. 677 - 692作者:Barluenga, Jose、Yus, Miguel、Concellon, Jose M.、Bernad, PabloDOI:——日期:——
-
Mousseron et al., Bulletin de la Societe Chimique de France, 1946, p. 629,632作者:Mousseron et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
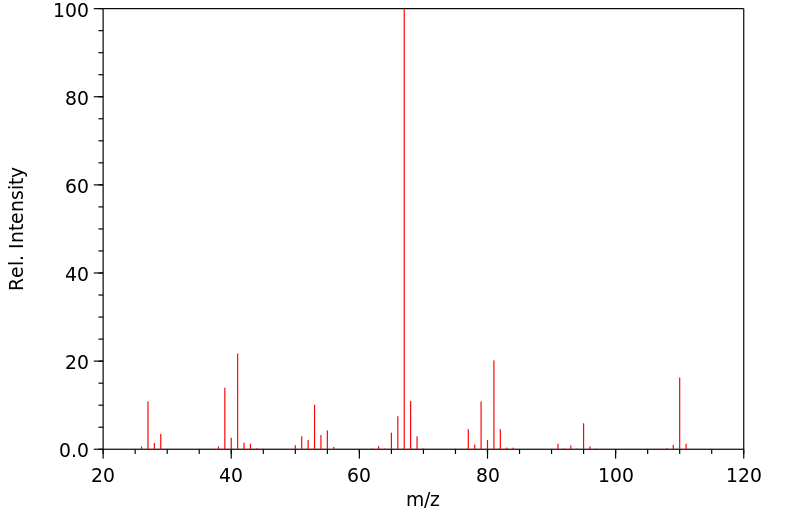
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-