N,N-二乙基二乙烯基三胺 | 24426-16-2
物质功能分类
中文名称
N,N-二乙基二乙烯基三胺
中文别名
1,1-二乙基二乙烯三胺;N,N-二乙基二亚乙基三胺
英文名称
N,N-diethyldiethylenetriamine
英文别名
N,N-Diethyl-diethylentriamin;1,1-Diethyldiethylenetriamine;N'-[2-(diethylamino)ethyl]ethane-1,2-diamine
CAS
24426-16-2
化学式
C8H21N3
mdl
——
分子量
159.275
InChiKey
CEFDTSBDWYXVHY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:225-230 °C(lit.)
-
密度:0.865 g/mL at 25 °C(lit.)
-
闪点:225-230°C
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。请避免接触氧化物、酸以及铜。
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:11
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:41.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S27,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2921290000
-
包装等级:III
-
危险类别:8
-
危险品运输编号:UN 2735 8/PG 2
-
储存条件:请确保贮藏器密封,并将其存放在阴凉、干燥的地方,最好放入一个紧密封装的容器中。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product name : N,N-Diethyldiethylenetriamine
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
According to Regulation (EC) No1272/2008
Skin corrosion (Category 1B)
According to European Directive 67/548/EEC as amended.
Causes burns.
Label elements
Pictogram
Signal word Danger
Hazard statement(s)
Causes severe skin burns and eye damage.
Precautionary statement(s)
Wear protective gloves/protective clothing/eye protection/face protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Immediately call a POISON CENTER or doctor/physician.
Hazard symbol(s)
C Corrosive
R-phrase(s)
R34 Causes burns.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S27 Take off immediately all contaminated clothing.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
S45 In case of accident or if you feel unwell, seek medical advice immediately
(show the label where possible).
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Formula : C8H21N3
Molecular Weight : 159,27 g/mol
CAS-No. EC-No. Index-No. Classification Concentration
N'-(2-Aminoethyl)-N,N-diethylethylenediamine
24426-16-2 246-242-2 - Skin Corr. 1B; H314 -
C, R34
For the full text of the H-Statements mentioned in this Section, see Section 16.
Section 4. FIRST AID MEASURES
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing give artificial respiration Consult a physician.
In case of skin contact
Take off contaminated clothing and shoes immediately. Wash off with soap and plenty of water. Consult a
physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water.
Consult a physician.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special protective equipment for fire-fighters
Wear self contained breathing apparatus for fire fighting if necessary.
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Evacuate personnel to safe areas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Soak up with inert absorbent material and dispose of as hazardous waste. Keep in suitable, closed
containers for disposal.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid inhalation of vapour or mist.
Normal measures for preventive fire protection.
Conditions for safe storage
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Personal protective equipment
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator with
multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup to
engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Hand protection
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the
standard EN 374 derived from it.
Handle with gloves.
Eye protection
Tightly fitting safety goggles. Faceshield (8-inch minimum).
Skin and body protection
Choose body protection according to the amount and concentration of the dangerous substance at the
work place.
Hygiene measures
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at
the end of workday.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance
Form liquid
Colour colourless
Safety data
pH no data available
Melting point no data available
Boiling point 225 - 230 °C - lit.
Flash point 95 °C - closed cup
Ignition temperature no data available
Lower explosion limit no data available
Upper explosion limit no data available
Density 0,865 g/cm3 at 25 °C
Water solubility no data available
Section 10. STABILITY AND REACTIVITY
Chemical stability
Stable under recommended storage conditions.
Conditions to avoid
no data available
Materials to avoid
Strong oxidizing agents
Hazardous decomposition products
Hazardous decomposition products formed under fire conditions. - Carbon oxides, nitrogen oxides (NOx)
Section 11. TOXICOLOGICAL INFORMATION
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Material is extremely destructive to the tissue of the
mucous membranes and upper respiratory tract.
Ingestion May be harmful if swallowed. Causes burns.
Skin May be harmful if absorbed through skin. Causes skin burns.
Eyes Causes eye burns.
Signs and Symptoms of Exposure
Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and
skin., spasm, inflammation and edema of the larynx, spasm, inflammation and edema of the bronchi,
pneumonitis, pulmonary edema, burning sensation, Cough, wheezing, laryngitis, Shortness of breath,
Headache, Nausea
Additional Information
RTECS: no data available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Product
Observe all federal, state, and local environmental regulations. Contact a licensed professional waste
disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
ADR/RID
UN-Number: 2735 Class: 8 Packing group: II
Proper shipping name: POLYAMINES, LIQUID, CORROSIVE, N.O.S. (N'-(2-Aminoethyl)-N,N-
diethylethylenediamine)
IMDG
UN-Number: 2735 Class: 8 Packing group: II EMS-No: F-A, S-B
Proper shipping name: POLYAMINES, LIQUID, CORROSIVE, N.O.S. (N'-(2-Aminoethyl)-N,N-
diethylethylenediamine)
Marine pollutant: No
IATA
UN-Number: 2735 Class: 8 Packing group: II
Proper shipping name: Polyamines, liquid, corrosive, n.o.s. (N'-(2-Aminoethyl)-N,N-diethylethylenediamine)
Section 15. REGULATORY INFORMATION
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二乙基乙二胺 N,N-diethylethylenediamine 100-36-7 C6H16N2 116.206
反应信息
-
作为反应物:描述:N,N-二乙基二乙烯基三胺 在 镍 一水合肼 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 13.5h, 生成 4-amino-1-[2-[2-(diethylamino)ethylamino]ethylamino]-10H-acridin-9-one参考文献:名称:抗肿瘤咪唑并rid啶酮的结构-活性关系:体内合成和抗白血病活性。摘要:几种新的5-氨基-6H-咪唑并[4,5,1-de] -ac啶-6-一个在苯环上带有OH,OCH3,CH3,叔丁基或OCH2O的5-氨基取代衍生物的合成组进行了描述。8-OH取代的化合物或双取代的7-OH-10-OCH3化合物具有抗鼠P388白血病的有效体内活性。5-[[2-[[2-[[((二乙基氨基)乙基]氨基]乙基]氨基] -8-羟基-6H-咪唑并[4,5,1-de] -rid啶-6-one显示最高的活性(4c)。DOI:10.1021/jm950564r
-
作为产物:描述:参考文献:名称:Hager; Al-Jaleel, Journal of the American Pharmaceutical Association (1912), 1955, vol. 44, p. 654摘要:DOI:
文献信息
-
USE OF NITROGEN COMPOUNDS QUATERNISED WITH ALKYLENE OXIDE AND HYDROCARBYL-SUBSTITUTED POLYCARBOXYLIC ACID AS ADDITIVES IN FUELS AND LUBRICANTS申请人:BASF SE公开号:US20160130514A1公开(公告)日:2016-05-12The invention relates to the use of quaternized nitrogen compounds as a fuel and lubricant additive or kerosene additive, such as in particular as a detergent additive, for decreasing or preventing deposits in the injection systems of direct-injection diesel engines, in particular in common rail injection systems, for decreasing the fuel consumption of direct-injection diesel engines, in particular of diesel engines having common rail injection systems, and for minimizing the power loss in direct-injection diesel engines, in particular in diesel engines having common rail injection systems; the invention further relates to the use as an additive for petrol, in particular for operation of DISI engines.该发明涉及将季铵化氮化合物用作燃料和润滑剂添加剂或煤油添加剂,特别是作为清洁剂添加剂,用于减少或预防直喷柴油发动机的喷射系统中的沉积物,在特定是在共轨喷射系统中,用于降低直喷柴油发动机的燃料消耗,特别是具有共轨喷射系统的柴油发动机,并用于减少直喷柴油发动机的功率损失,特别是在具有共轨喷射系统的柴油发动机中;该发明还涉及将其用作汽油添加剂,特别是用于DISI发动机的运行。
-
Novel lipophilic chloroquine analogues for a highly efficient gene transfer into gynecological tumors作者:Oliver Keil、Hans Bojar、Hans-Bernd Prisack、Peter DallDOI:10.1016/s0960-894x(01)00516-9日期:2001.10cationic lipids have been proven to be an attractive alternative to viral vectors in gene therapy protocols with regard to safety and manufacturing concerns. In order to improve the transfection efficiency we have synthesized two novel carboxycholesteryl-modified chloroquine analogues. Due to their potential endosomal buffering capacity these compounds enable the efficient transfection of various gynecological
-
[EN] DIUREA DERIVATIVES<br/>[FR] DERIVES DE DIUREE申请人:ACTIVE BIOTECH AB公开号:WO2005074919A1公开(公告)日:2005-08-18The present invention relates to novel diurea derivatives that block intracellular signal transduction and thereby inhibit the production of pro-inflammatory cytokines, especially interleukin-2 (IL-2) and/or induce apoptosis in activated T-cells. It further discloses such a compound for use as a medicament, the use of said compound for the manufacturing of a medicament for the treatment of immune disorders which benefit from inhibition of production of IL-2 and other pro-inflammatory cytokines and/or induction of apoptosis in activated T-cells, a pharmaceutical composition comprising said compound and a method of treatment comprising administration of a pharmaceutically effective amount of said compound. A compound of the general formula I.
-
The first report of a tetra-azide bound mononuclear cobalt(<scp>iii</scp>) complex and its comparative biomimetic catalytic activity with tri-azide bound cobalt(<scp>iii</scp>) compounds作者:Narayan Ch. Jana、Paula Brandão、Anangamohan PanjaDOI:10.1039/d0nj02339f日期:——centre in 1 is bonded with four terminal azide ions and two donor sites of triamine L1, leaving the tertiary amine group protonated. All the complexes are stabilized by rich hydrogen bonding interactions, leading to hydrogen bonded supramolecular chain structures. It is worth noting that complex 1 is the first example in cobalt(III) coordination chemistry in which all four azide ions coordinate the三种新的叠氮化物键合钴(III)配合物[Co(HL 1)(N 3)4 ](1),[Co(L 2)(N 3)3 ](2)和[Co(L 3)( N 3)3 ](3),其中L 1,L 2和L 3为N,N-二甲基二丙烯三胺,N 1-异丙基二亚乙基三胺和N,N分别合成了-二乙基二亚乙基三胺并进行了结构表征。X射线晶体学研究表明,2和3的结构非常相似,其中三个末端叠氮化物离子与三胺一起协调金属中心。复杂1,另一方面是从作为金属中心的另外两个显著不同1键合具有四个末端叠氮离子和三胺升2个供体部位1,留下质子化的叔胺基团。所有的配合物都通过丰富的氢键相互作用而稳定,从而形成氢键超分子链结构。值得注意的是,络合物1是钴的第一个例子(III)配位化学,其中所有四个叠氮化物离子末端配位金属中心。所有这些化合物在好氧条件下对邻氨基酚与苯恶嗪酮发色团的氧化偶合均表现出有效的催化活性,并探索了结构因素在催化活性中的作
-
[EN] LIPID-LIKE NANOCOMPLEXES AND USES THEREOF<br/>[FR] NANOCOMPLEXES DE TYPE LIPIDE ET LEURS UTILISATIONS申请人:TUFTS COLLEGE公开号:WO2019152848A1公开(公告)日:2019-08-08Disclosed are compounds of formula (I) below: (I), wherein each of the variables A, B, X, W, V, R1-R5, and m is defined herein. Also disclosed are pharmaceutical compositions containing a nanocomplex, wherein the nanocomplex is formed of one of the compounds, and a protein, a nucleic acid, or a small molecule; and methods of treating a medical condition with one of the pharmaceutical compositions.以下是公式(I)的化合物:(I),其中在此定义了变量A、B、X、W、V、R1-R5和m的每个变量。还公开了含有纳米复合物的药物组合物,其中纳米复合物由化合物之一和蛋白质、核酸或小分子形成;以及使用其中一种药物组合物治疗医疗状况的方法。
表征谱图
-
氢谱1HNMR
-
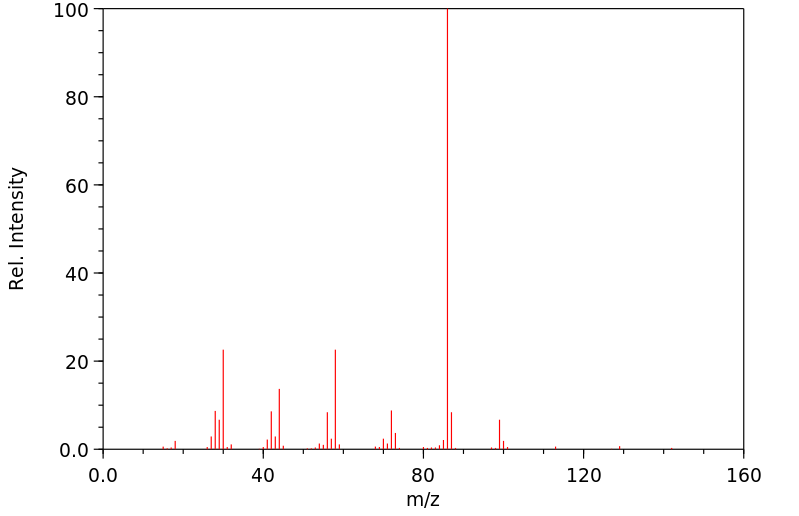
质谱MS
-
碳谱13CNMR
-
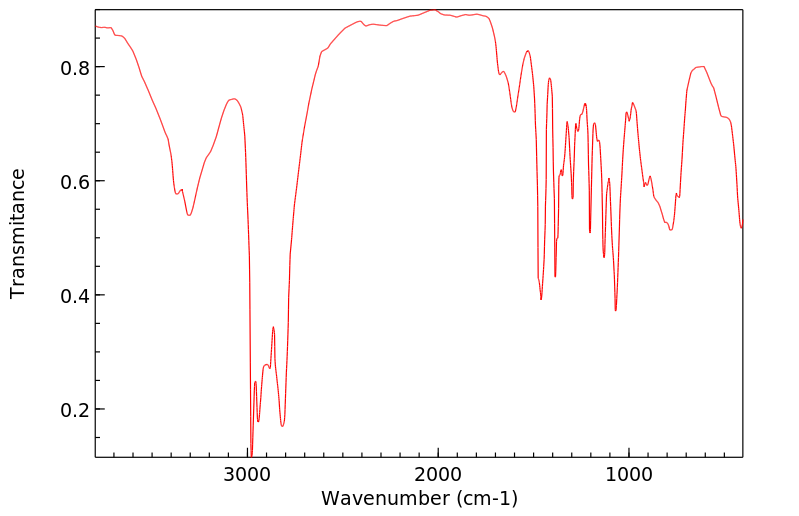
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷