苄基月桂酸酯 | 6939-75-9
物质功能分类
中文名称
苄基月桂酸酯
中文别名
苄基乙酰丙酸
英文名称
benzyl levulinate
英文别名
benzyl 4-oxopentanoate;Laevulinsaeurebenzylester
CAS
6939-75-9
化学式
C12H14O3
mdl
MFCD00062778
分子量
206.241
InChiKey
KWQUVANYFZOCEA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:305.09°C (rough estimate)
-
密度:1.0935
-
LogP:1.62
-
物理描述:Viscous oil to waxy solid; Strong fruity aroma
-
溶解度:Practically insoluble or insoluble in water
-
折光率:1.503-1.509
-
保留指数:2526
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:15
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2918300090
SDS
| Name: | Benzyl levulinate 96%(GC) Material Safety Data Sheet |
| Synonym: | Benzyl 4-oxovalerat |
| CAS: | 6939-75-9 |
Synonym:Benzyl 4-oxovalerat
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6939-75-9 | Benzyl levulinate | 96 | 230-075-7 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Cover with sand, dry lime or soda ash and place in a closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6939-75-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: 1.0730g/cm3
Molecular Formula: C12H14O3
Molecular Weight: 206.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6939-75-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Benzyl levulinate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 6939-75-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6939-75-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6939-75-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙酰丙酮苄酯 benzyl acetoacetate 5396-89-4 C11H12O3 192.214 丙烯酸苄酯 benzylacrylate 2495-35-4 C10H10O2 162.188 碘乙酸苄基酯 benzyl iodoacetate 81867-37-0 C9H9IO2 276.074 2-溴乙酸苄酯 Benzyl bromoacetate 5437-45-6 C9H9BrO2 229.073 —— benzyl diazoacetate 52267-51-3 C9H8N2O2 176.175 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丙酸苄酯 benzyl proprionate 122-63-4 C10H12O2 164.204 —— benzyl 4,4-difluoropentanoate 1581718-70-8 C12H14F2O2 228.239 丙烯酸苄酯 benzylacrylate 2495-35-4 C10H10O2 162.188
反应信息
-
作为反应物:描述:苄基月桂酸酯 在 palladium on activated charcoal 氢气 作用下, 生成 1-(1-adamantoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid参考文献:名称:Boltze; Brendler; Jacobi, Arzneimittel-Forschung/Drug Research, 1980, vol. 30, # 8 A, p. 1314 - 1325摘要:DOI:
-
作为产物:描述:2-溴-1-乙基吡啶四氟硼酸盐 以45%的产率得到参考文献:名称:SAIGO K.; USUI M.; KIKUCHI K.; SHIMADA E.; MUKAIYAMA T., BULL. CHEM. SOC. JAP.
, 1977, 50, NO 7, 1863-1866 摘要:DOI:
文献信息
-
Discriminating non-ylidic carbon-sulfur bond cleavages of sulfonium ylides for alkylation and arylation reactions作者:Jing Fang、Ting Li、Xiang Ma、Jiuchang Sun、Lei Cai、Qi Chen、Zhiwen Liao、Lingkui Meng、Jing Zeng、Qian WanDOI:10.1016/j.cclet.2021.06.069日期:2022.1The disparate reaction pattern allowed the separate activation of non-ylidic S-alkyl and S-aryl bond. Under acidic conditions, sulfonium ylides serve as alkyl cation precursors which facilitate the alkylations. While under alkaline conditions, cleavage of non-ylidic S-aryl bond produces O-arylated compounds efficiently. The robustness of the protocols were established by the excellent compatibility of
-
[EN] FLUOROALKYL AND FLUOROCYCLOALKYL 1,4-BENZODIAZEPINONE COMPOUNDS AS NOTCH|INHIBITORS<br/>[FR] COMPOSÉS DE FLUORALKYL- ET DE FLUOROCYCLOALKYL-1,4-BENZODIAZÉPINONE UTILISABLES EN TANT QU'INHIBITEURS DU RÉCEPTEUR NOTCH申请人:BRISTOL MYERS SQUIBB CO公开号:WO2014047397A1公开(公告)日:2014-03-27Disclosed are compounds of Formula (I): (Formula (I)) wherein: R1 is -CH2CH2CF3; R2 is -CH2CH2CH2F, -CH2CF2CH3, -CH2CH2CF3, -CH2CH(CH3)CF3, -CH2CH2CF2CH3, (Formulae (II), (III), (IV), (V) or (VI): R3 is H or -CH3; Ring A is phenyl or pyridinyl; and Rx, Ry, Ra, Rb, y, and z are defined herein. Also disclosed are methods of using such compounds to inhibit the Notch receptor, and pharmaceutical compositions comprising such compounds. These compounds are useful in treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as cancer.
-
Synthesis of 1,4-Dicarbonyl Compounds from Silyl Enol Ethers and Bromocarbonyls, Catalyzed by an Organic Dye under Visible-Light Irradiation with Perfect Selectivity for the Halide Moiety over the Carbonyl Group作者:Naoto Esumi、Kensuke Suzuki、Yoshihiro Nishimoto、Makoto YasudaDOI:10.1021/acs.orglett.6b02869日期:2016.11.4We report the visible-light-induced radical coupling reaction of silyl enol ethers with α-bromocarbonyl compounds to give 1,4-dicarbonyls. The reaction was effectively accelerated using an inexpensive organic dye (eosin Y) as a photoredox catalyst. 1,4-Dicarbonyl compounds alone were afforded, without the generation of carbonyl adducts of the α-halocarbonyls, which are usually generated in the presence
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
一种1,4-二羰基化合物类衍生物的光化学合成方法
表征谱图
-
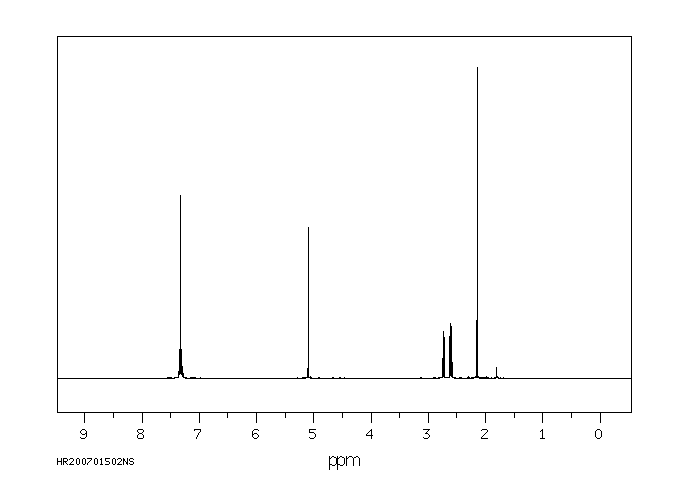
氢谱1HNMR
-
质谱MS
-
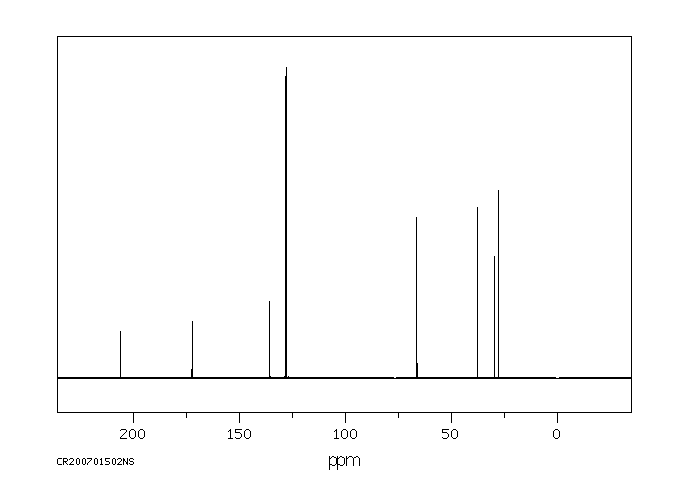
碳谱13CNMR
-
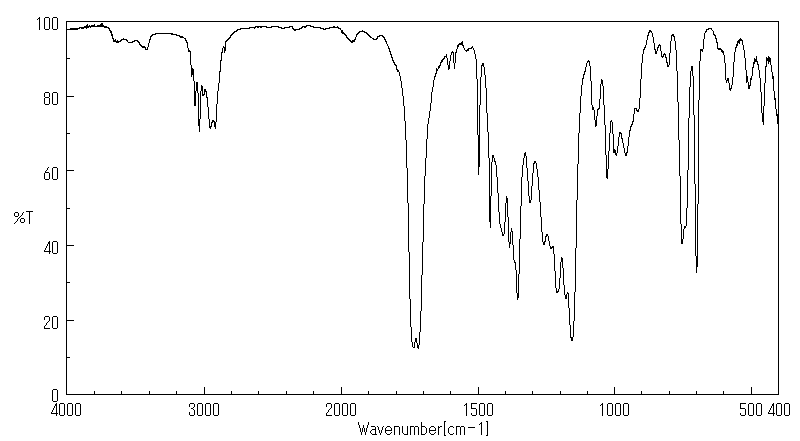
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫