氨鲁米特 | 125-84-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:152-154 °C(lit.)
-
沸点:374.44°C (rough estimate)
-
密度:1.1099 (rough estimate)
-
溶解度:H2O:0.2 mg/mL,微溶
-
物理描述:Solid
-
颜色/状态:Cyrstals from methanol or ethyl acetate
-
蒸汽压力:4.96X10-10 mm Hg at 25 °C (est)
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
碰撞截面:164.8 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:2227
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.384
-
拓扑面积:72.2
-
氢给体数:2
-
氢受体数:3
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2925190090
-
危险品运输编号:3249
-
危险类别:6.1(b)
-
RTECS号:MA4026950
-
包装等级:III
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:存储条件:2-8°C,密闭保存。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : DL-Aminoglutethimide
CAS-No. : 125-84-8
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Skin irritation (Category 2)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Irritating to eyes, respiratory system and skin.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R36/37/38 Irritating to eyes, respiratory system and skin.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S36 Wear suitable protective clothing.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : 3-(p-Aminophenyl)-3-ethylpiperidine-2,6-dione
3-(4-Aminophenyl)-3-ethyl-2,6-piperidinedione
Formula : C13H16N2O2
Molecular Weight : 232,28 g/mol
Component Concentration
Aminoglutethimide
CAS-No. 125-84-8 -
EC-No. 204-756-4
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Immersion protection
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: > 480 min
Material tested:Dermatril® ( Z677272, Size M)
Splash protection
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: > 30 min
Material tested:Dermatril® ( Z677272, Size M)
data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 873000, test method: EN374
If used in solution, or mixed with other substances, and under conditions which differ from EN 374,
contact the supplier of the CE approved gloves. This recommendation is advisory only and must
be evaluated by an Industrial Hygienist familiar with the specific situation of anticipated use by our
customers. It should not be construed as offering an approval for any specific use scenario.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: powder
Colour: off-white
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 152 - 154 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
LD50 Intraperitoneal - mouse - 625 mg/kg
Remarks: Behavioral:Somnolence (general depressed activity). Behavioral:Antipsychotic.
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Damage to fetus possible
Developmental Toxicity - Human - female - Oral
Specific Developmental Abnormalities: Urogenital system.
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion
May be harmful if swallowed.
Skin May be harmful if absorbed through skin. Causes skin irritation.
Eyes
Causes serious eye irritation.
Additional Information
RTECS: MA4026950
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
氨鲁米特又名氨基导眠能,为芳香化酶抑制剂,是格鲁米特(导眠能)的衍生物。它通过阻断芳香化酶而抑制雌激素生成,减少雌激素对乳腺癌的促进作用,从而起到抑制肿瘤生长的效果。
此外,氨鲁米特可在肾上腺皮质和腺体外组织两个不同部位阻断雄激素的生物合成,产生药物性肾上腺切除作用。在腺体内,它主要阻止胆固醇转变为孕烯醇酮,从而抑制肾上腺皮质中自体激素的生物合成;在周围组织中则具有强力的芳香化酶抑制作用,阻止雄激素转变为雌激素。绝经后妇女的雌激素主要来源是雄烯二酮在脂肪、肌肉和肝脏中的芳香化转变,而氨鲁米特对这一过程的抑制效果比对肾上腺皮质激素合成的抑制强10倍。垂体后叶分泌的ACTH能对抗氨鲁米特抑制肾上腺皮质激素合成的作用,因此使用本品时可合用氢化可的松以阻滞这种作用。
该药物主要治疗晚期乳腺癌(绝经后及雌激素受体阳性者疗效较好)、卵巢癌、前列腺癌和肾上腺皮质癌。此外,它也用于皮质醇增多症(库欣综合征)及因肾上腺肿瘤引起类似症状的情况。
药动学氨鲁米特口服后胃肠道吸收迅速而完全,生物利用度约为75%,1.5小时血药浓度达峰值。长期高剂量(每日500mg)口服时,血药峰浓度(Cmax)平均为9μg/ml;长期低剂量(每日125~250mg)口服时,Cmax为0.5~1.5μg/ml。单次用药半衰期约为12.5小时,连续给药2周后,半衰期缩短至6~7小时。血浆蛋白结合率为20%~25%,主要在肝脏代谢,代谢产物主要为N-乙酰氨鲁米特。药物以原形和代谢产物形式主要通过尿液排出,少量从胆汁中排出。
不良反应- 神经系统:嗜睡、眩晕、共济失调、眼球震颤。
- 消化系统:食欲不振、恶心、呕吐、便秘、腹泻以及肝炎。
- 皮肤:皮疹、瘙痒,极罕见剥脱性皮炎、Stevens-Johnson综合征、Lyell综合征。
- 呼吸系统:过敏性肺炎。
- 血液系统:中性粒细胞减少、血小板减少,甚至全血细胞减少,偶有粒细胞缺乏。
- 内分泌系统:个别出现肾上腺或甲状腺功能减退、女性男性化等。
- 对本药过敏者禁用。
- 卟啉病患者禁用。
- 儿童禁用。
- 带状疱疹或其他感染性疾病患者禁用。
- 肾上腺皮质功能减退症患者禁用。
- 肝、肾功能不全者禁用。
口服:每次250mg,每日2次,两周后改为每日3~4次,每日量不超过1g。用药同时应服用氢化可的松40mg(早晨及下午5点各10mg,临睡前20mg),以防止因肾上腺皮质产生氢化可的松减少而引起脑垂体对肾上腺皮质激素的反馈性增加。
生产方法由2-对氯苯基丁酰胺为原料制得。
类别有毒物品
毒性分级中毒
急性毒性腹腔-小鼠LD50: 625毫克/公斤
可燃性危险特性可燃;燃烧产生有毒氮氧化物烟雾
储运特性库房通风低温干燥
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 格鲁米特 Glutethimide 77-21-4 C13H15NO2 217.268 3-乙基-3-(4-硝基苯基)-2,6-哌啶二酮 rac-3-ethyl-3-(4-nitrophenyl)piperidine-2,6-dione 38527-73-0 C13H14N2O4 262.265 1-苄氧基-3-乙基-3-(4-硝基苯基)哌啶-2,6-二酮 1-benzyloxy-3-ethyl-3-(4-nitrophenyl)piperidine-2,6-dione 183663-75-4 C20H20N2O5 368.389 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (R)-(+)-氨鲁米特 (R)-(+)-aminoglutethimide 55511-44-9 C13H16N2O2 232.282 (S)-(-)-氨鲁米特 aminoglutethimide 57288-03-6 C13H16N2O2 232.282 3-乙基-3-[4-(甲基氨基)苯基]哌啶-2,6-二酮 3-ethyl-3-(4-(methylamino)phenyl)piperidine-2,6-dione 62268-20-6 C14H18N2O2 246.309 格鲁米特 Glutethimide 77-21-4 C13H15NO2 217.268 N-[4-(3-乙基-2,6-二氧代哌啶-3-基)苯基]乙酰胺 N-(4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl)acetamide 38473-28-8 C15H18N2O3 274.32 —— N-methyl-3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione 52498-52-9 C14H18N2O2 246.309 —— 2-Ethyl-2-(4-hydroxyphenyl)-glutarimid 50275-56-4 C13H15NO3 233.267 —— p-amino-m-hydroxyglutethimide 83417-19-0 C13H16N2O3 248.282 —— 1,3-bis[4-(3-ethyl-2,6-dioxo-3-piperidinyl)-phenyl]-triazene —— C26H29N5O4 475.547 3-(4-氨基-3-碘苯基)-3-乙基哌啶-2,6-二酮 o-iodoaminoglutethimide 61435-64-1 C13H15IN2O2 358.179 —— 3-[4-(N,N-dimethylsulfamoyl)phenyl]-3-ethyl-2,6-piperidinedione —— C15H21N3O4S 339.415 —— N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]-1-phenylmethanesulfonamide —— C20H22N2O4S 386.472 —— N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]-4-methylbenzenesulfonamide —— C20H22N2O4S 386.472 —— 4-amino-N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]benzenesulfonamide 201336-47-2 C19H21N3O4S 387.459 —— 4-bromo-N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]benzenesulfonamide —— C19H19BrN2O4S 451.341 —— 4-chloro-N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]benzenesulfonamide —— C19H19ClN2O4S 406.89 —— N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]-4-fluorobenzenesulfonamide —— C19H19FN2O4S 390.435 —— 3-amino-N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]benzenesulfonamide 201336-48-3 C19H21N3O4S 387.459 —— N-(4-{[4-(3-ethyl-2,6-dioxo-3-piperidyl)anilino]sulfonyl}phenyl)acetamide —— C21H23N3O5S 429.497 —— 1-(3,4-Dichlorophenyl)-3-[4-[[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]sulfamoyl]phenyl]urea —— C26H24Cl2N4O5S 575.472 —— 2-[4-(3-Ethyl-2,6-dioxopiperidin-3-yl)phenyl]isoindole-1,3-dione —— C21H18N2O4 362.385 —— N-<4-(3-ethyl-2,6-dioxo-3-piperidyl)phenyl>-5-dimethylamino-1-naphthalenesulfonamide —— C25H27N3O4S 465.573 —— 3-ethyl-3-{4-[4-(tosylsulfonylureido)phenylsulfamoyl]phenyl}-2,6-piperidinedione —— C27H28N4O7S2 584.674 —— 3-[3-(3,4-Dichloro-phenyl)-ureido]-N-[4-(3-ethyl-2,6-dioxo-piperidin-3-yl)-phenyl]-benzenesulfonamide —— C26H24Cl2N4O5S 575.472 —— N-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenyl]-2-nitrobenzenesulfonamide —— C19H19N3O6S 417.442 —— (S)-2-[4-(3-ethyl-2,6-dioxopiperidin-3-yl)phenylcarbamoyl]pyrrolidine-1-carboxylic acid benzyl ester —— C26H29N3O5 463.533 —— 3-ethyl-3-{3-[4-(tosylsulfonylureido)phenylsulfamoyl]phenyl}-2,6-piperidinedione —— C27H28N4O7S2 584.674 —— 2-[4-(3-Ethyl-2,6-dioxopiperidin-3-yl)phenyl]-5,6-dimethoxyisoindole-1,3-dione —— C23H22N2O6 422.437 —— 2-[4-(3-Ethyl-2,6-dioxo-3-piperidyl)phenyl]-5-nitro-benzo[de]isoquinoline-1,3-dione —— C25H19N3O6 457.442 —— 3-ethyl-3-(4-{(Z)-[(2-oxo-2,3-dihydro-1H-pyrrolo[3,2-f]quinolin-1-ylidene)methyl]amino}phenyl)-2,6-piperidinedione 297756-95-7 C25H22N4O3 426.475 - 1
- 2
- 3
反应信息
-
作为反应物:描述:氨鲁米特 在 ethyl 2-((adamantan-1-yl)amino)-2-oxoacetate 、 氧气 、 silver(I) acetate 、 palladium diacetate 、 三乙胺 作用下, 以 二氯甲烷 为溶剂, 反应 24.0h, 生成 N-(4-(3-ethyl-2,6-dioxopiperidin-3-yl)-2-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodec-1-en-1-yl)phenyl)pivalamide参考文献:名称:配体促进的通过苯甲酰胺间接引入氟化官能团的苯甲酸酯的烯烃化摘要:我们报告了以4-溴-3,3,4,4-四氟丁烯为氟化试剂的钯催化的,配体促进的,苯甲酰化的C–H含氟烯烃的氟化反应,由于其- CF 2 CF 2 Br官能团。- CF 2 CF 2 H为通过使用温和的还原剂硼氢化钠获得。生物活性化合物,例如氨基戊二酰亚胺衍生物和丙胺在该反应中具有良好的耐受性,这两者都突出了该方法的合成重要性。DOI:10.1021/acs.joc.0c02701
-
作为产物:描述:参考文献:名称:钴催化醚的直接氨基羰基化:高效获得 α-酰胺取代的醚衍生物摘要:已经实现了醚与胺的新型钴催化羰基化偶联以构建α-羰基化醚。阿夫唑嗪是一种治疗良性前列腺增生(BPH)的药物,可以通过这个过程直接合成。DOI:10.1002/anie.202203797
-
作为试剂:描述:1-氧代-5-氮杂螺[2.3]-5-羧酸叔丁酯叔丁基 、 吗啉 在 乙酸乙酯 、 水 、 Brine 、 Sodium sulfate-III 、 silica gel 、 三氟乙酸 、 氨鲁米特 、 hydroxide 作用下, 以 四氢呋喃 为溶剂, 反应 1.08h, 以afforded 3-(morpholin-4-ylmethyl)azetidin-3-ol (11.6 mg, 24% yield) as a colorless oil的产率得到3-(Morpholin-4-ylmethyl)azetidin-3-ol参考文献:名称:Azetidines as MEK Inhibitors for the Treatment of Proliferative Diseases摘要:本发明公开了式(I)的化合物及其药学上可接受的盐和溶剂化物。这些化合物是MEK抑制剂,可用于治疗增生性疾病,如癌症。本发明还公开了含有这些化合物的制药组合物,以及使用本发明的化合物和组合物治疗癌症的方法。公开号:US20100249096A1
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] COMPOUNDS AND COMPOSITIONS COMPRISING CDK INHIBITORS AND METHODS FOR THE TREATMENT OF CANCER<br/>[FR] COMPOSÉS ET COMPOSITIONS COMPRENANT DES INHIBITEURS DES CDK ET MÉTHODES DE TRAITEMENT DU CANCER申请人:UNIV GEORGIA STATE RES FOUND公开号:WO2010129858A1公开(公告)日:2010-11-11Disclosed herein are compounds suitable for use as antitumor agents, methods for treating cancer wherein the disclosed compounds are used in making a medicament for the treatment of cancer, methods for treating a tumor comprising, administering to a subject a composition comprising one or more of the disclosed cytotoxic agents, and methods for preparing the disclosed antitumor agents.
-
Cobalamin conjugates for anti-tumor therapy申请人:Weinshenker M. Ned公开号:US20050054607A1公开(公告)日:2005-03-10The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.本发明提供了一种适用于治疗肿瘤相关疾病的钴胺素-药物结合物。钴胺素通过可切割的连接剂间接共价结合到抗肿瘤药物上,还可以通过一个或多个可选的间隔物。钴胺素通过其核糖环的5'-OH与第一间隔物或可切割连接剂共价结合。药物通过其现有或添加的功能基团与可切割连接剂的第二间隔物结合。在给药后,结合物与转钴胺素(其任何同工异构体)形成复合物。然后,该复合物结合到细胞膜上的受体并被细胞摄取。一旦进入细胞,细胞内酶将切割结合物,从而释放药物。根据结合物的结构,特定类别或类型的细胞内酶影响切割。由于生长细胞对钴胺素的需求量较高,肿瘤细胞通常摄取结合物的比例高于正常非生长细胞。本发明的结合物与相应的游离药物相比,具有较低的全身毒性和增强的疗效。
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOCAD JOINT STOCK CO公开号:WO2018092047A1公开(公告)日:2018-05-24The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.本发明涉及一种新的化合物,其化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为CH2,NH,O或化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[化学式II]每个R7,R8,R9,R10独立地为乙烯基,甲基乙炔基;Hal为CI,Br,I,F,具有布鲁顿酪氨酸激酶(Btk)抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。
表征谱图
-
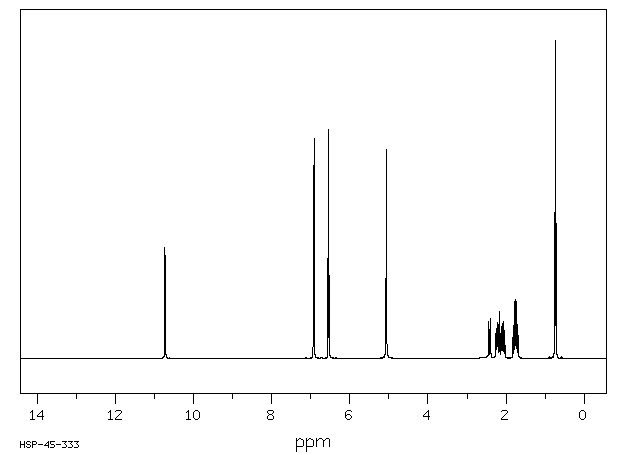
氢谱1HNMR
-
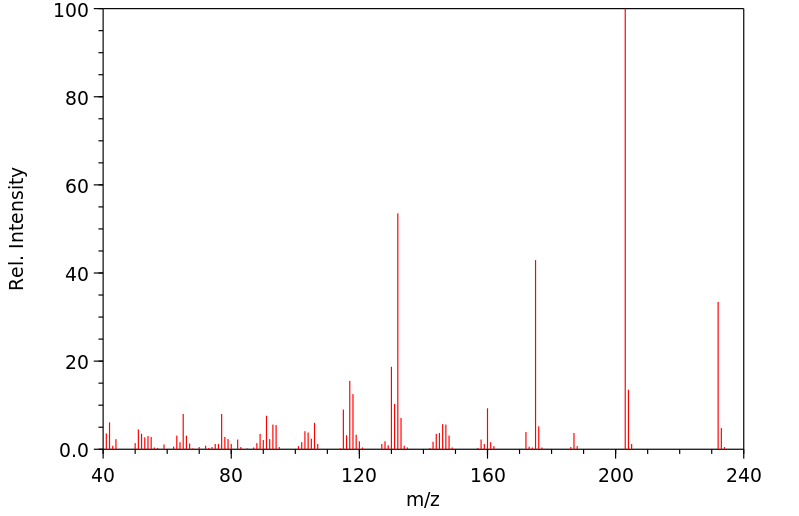
质谱MS
-
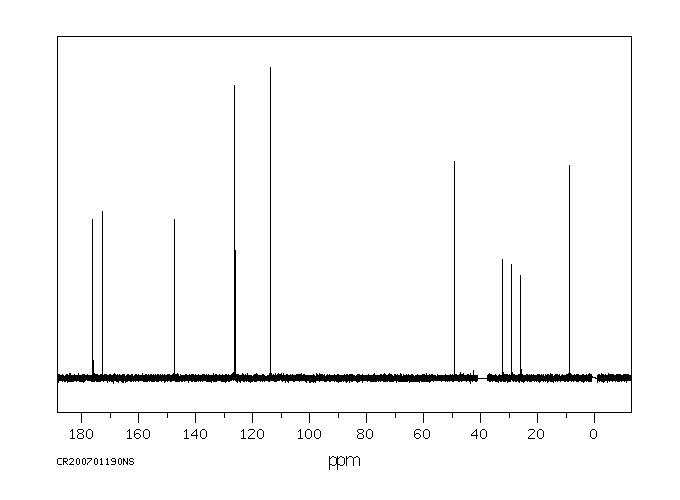
碳谱13CNMR
-
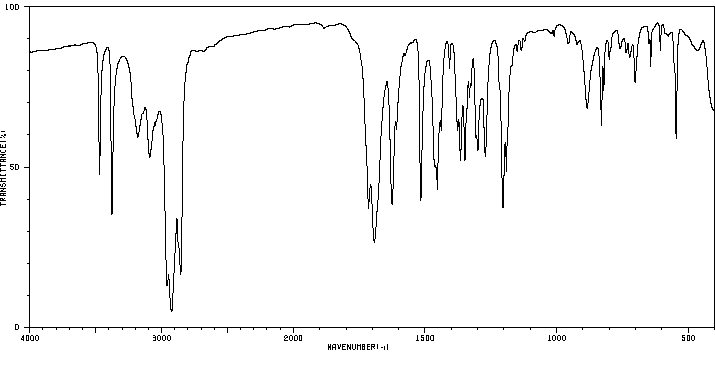
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息