丙烯腈 | 107-13-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-83 °C (lit.)
-
沸点:77 °C (lit.)
-
密度:0.806 g/mL at 20 °C
-
蒸气密度:1.83 (vs air)
-
闪点:32 °F
-
溶解度:水中的溶解度为73克/升
-
介电常数:33.009999999999998
-
暴露限值:NIOSH REL: TWA 1 ppm, 15-min C 1 ppm, IDLH 85 ppm; OSHA PEL: TWA 2 ppm, 15-min C 10 ppm; ACGIH TLV: TWA 2 ppm.
-
LogP:0.017 at 21℃
-
物理描述:Acrylonitrile, stabilized appears as a clear colorless liquid with a strong pungent odor. Flash point 32°F. Prolonged exposure to the vapors or skin contact harmful. Density 6.7 lb / gal. Vapors heavier than air. Combustion produces toxic oxides of nitrogen. Requires storage and handling in closed systems. Used in insecticides and to make plastics, fibers and other chemicals. Rate of onset: Immediate Persistence: Minutes to hours Odor threshold: 17 ppm Source/use/other hazard: Plastics, coatings, adhesives industries; dyes; pharmaceuticals; flam gas.
-
颜色/状态:Clear, colorless liquid at room temperature
-
气味:Practically odorless, or with a very slight odor of peach kernels
-
蒸汽密度:1.9 (EPA, 1998) (Relative to Air)
-
蒸汽压力:109 mm Hg at 25 °C
-
亨利常数:1.38e-04 atm-m3/mole
-
大气OH速率常数:4.10e-12 cm3/molecule*sec
-
稳定性/保质期:
-
化学性质:化学性质活泼,能发生双键加成反应,与相应的含有活泼氢的无机或有机化合物反应生成一系列氰乙基化产物。在缺氧或可见光照射下易聚合,在浓碱中则会强烈聚合。与还原剂反应剧烈,释放有毒气体。其蒸气与空气混合可形成爆炸性混合物,并能与氧化剂发生强烈反应,遇明火、高温时有燃烧爆炸的危险。在光照、受热或久贮情况下也会容易聚合,存在燃烧和爆炸的风险。
-
本品极为毒害,对温血动物的毒性约为氰化氢的1/30。丙烯腈不仅蒸气有毒性,皮肤接触也会中毒。小鼠静脉注射LD50值为15mg/kg,大鼠的LD50值是93mg/kg。长时间吸入稀丙烯腈蒸气会导致恶心、呕吐、头痛、疲劳和不适等症状。工作场所允许的最大浓度不超过45mg/m³。操作时要确保设备密封,并佩戴防护用品;如不慎沾染衣物或皮肤,应立即脱掉衣物并用大量清水冲洗;若溅入眼睛,则需用水冲洗15分钟以上;误食时,应用温盐水洗胃。如发生中毒情况,请立即使用硫代硫酸钠和亚硝酸钠进行静脉注射,并寻求医生帮助。
-
稳定性:稳定
-
禁配物:强氧化剂、碱类、酸类
-
应避免接触的条件:受热、光照及与空气接触
-
聚合危害:聚合
-
分解产物:氰化氢
-
-
自燃温度:898 °F (481 °C)
-
分解:PYROLYSIS OF ORLON @ 600 °C IN NITROGEN ATMOSPHERE GIVES RELATIVELY LARGE AMT OF HYDROGEN CYANIDE, AMMONIA, HYDROGEN, & METHANE ... .
-
粘度:0.34 sq mm/s at 25 °C
-
腐蚀性:Attacks copper and copper alloys ... attacks aluminum in high concentration
-
燃烧热:1761.8 kJ/mol at 25 °C (liquid)
-
汽化热:32.6 kJ/mol at 25 °C
-
表面张力:27.76 dyn/cm at 15.1 °C; 27.54 mN/m at 17.8 °C
-
电离电位:10.91 eV
-
聚合:Hazardous polymerization may occur. Polymerization may be caused by elevated temperature or alkalies. Uninhibited monomer vapor may form polymer in vents and other confined spaces.
-
气味阈值:Detection of acrylonitrile in water is 1.86x10+1 ppm; chemically pure
-
折光率:Index of refraction: 1.3911 at 20 °C/D
-
相对蒸发率:4.54 (Butyl acetate = 1)
-
保留指数:520;496.4;500;491;495;499.1;493.4;495;492;495;490;492;516
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
职业暴露等级:D
-
职业暴露限值:TWA: 1 ppm, Ceiling: 10 ppm [15-minute] [skin]
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:60 ppm
-
危险品标志:Xn,F,T,N
-
安全说明:S16,S36/37,S45,S53,S61,S9
-
危险类别码:R51/53,R63,R43,R45,R11,R37/38,R23/24/25,R62,R39/23/24/25,R41
-
WGK Germany:3
-
海关编码:2926100000
-
危险品运输编号:UN 1093 3/PG 1
-
危险类别:3
-
RTECS号:AT5250000
-
包装等级:I
-
储存条件:1. **储存注意事项**:通常商品含有稳定剂。应将其储存在阴凉、通风良好的专用库房内,并实行“双人收发、双人保管”的管理制度。远离火源和热源,库温不宜超过37℃。包装需密封,避免与空气接触。应与氧化剂、酸类、碱类以及食用化学品分开存放,切忌混储。不宜大量储存或长期存放。使用防爆型照明和通风设施,并禁止使用可能产生火花的机械设备和工具。储存区应配备泄漏应急处理设备和合适的收容材料。 2. **包装及运输**:本品采用铁桶包装。贮存容器需密封,仓库要保持良好通风并避免日晒。远离强酸性物质如硫酸、硝酸等。按“危险品规定”进行储运。
SDS
| 国标编号: | 32162 |
| CAS: | 107-13-1 |
| 中文名称: | 丙烯腈 |
| 英文名称: | acrylonitrile;cyanoethylene |
| 别 名: | 乙烯基氰;氰(基)乙烯 |
| 分子式: | C 3 H 3 N;CH 2 CHCN |
| 分子量: | 53.06 |
| 熔 点: | -83.6℃ 沸点:77.3℃ |
| 密 度: | 相对密度(水=1)0.81; |
| 蒸汽压: | -5℃ |
| 溶解性: | 微溶于水,易溶于多数有机溶剂 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体,有桃仁气味 |
| 危险标记: | 7(易燃液体),40(有毒品) |
| 用 途: | 用于制造聚丙烯腈、丁腈橡胶、染料、合成树脂、医药等 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:本品在体内析出氰根,抑制呼吸酶;对呼吸中枢有直接麻醉作用。急性中毒表现与氢氰酸相似。
急性中毒:以中枢神经系统症状为主,伴有上呼吸道和眼部刺激症状。轻度中毒有头晕、头木、意识蒙胧及口唇紫绀等。眼结膜及鼻、咽部充血。重者除上述症状加重外,出现四肢阵发性强直抽搐、昏迷。液体污染皮肤,可致皮炎,局部出现红斑、丘疹或水疱。
二、毒理学资料及环境行为
毒性:属高毒类。
急性毒性:LD 50 78mg/kg(大鼠经口);250mg/kg(兔经皮);人吸入300~500mg/m 3 ×5~10分钟,上呼吸道灼痛、流泪;人吸入35~200mg/m 3 ×20~45分钟,粘膜刺激。
亚急性和慢性毒性:大鼠吸入40mg/m 3 ×4小时/日×6日/周×40日,致死,肝坏死;大鼠经口0.1%饮水×13周,生长减慢,萎靡。
刺激性:家兔经眼:20mg(24小时),重度刺激。家兔经皮:500mg,轻度刺激。
致突变性:微生物致变突性:鼠伤寒沙门氏菌25µL/皿。哺乳动物体细胞突变性:人淋巴细胞25mg/L。
生殖毒性:大鼠经口最低中毒剂量(TDL 0 ):650mg/kg(孕6~15天),对雄性生育指数有影响,可引起胚胎毒性,肌肉骨骼发育异常。
致癌性:大鼠经口最小中毒剂量1700mg/kg(37周)胃癌。
污染来源:丙烯腈是重要的有机原料,主要用于橡胶合成(如丁腈橡胶)、塑料合成(如ABS,AS树脂、聚丙烯酰胺等)、有机合成、制造腈纶、尼龙66等膈成纤维、杀虫剂、抗水剂、粘合剂等;还用合适谷物烟薰剂。从事丙烯腈生产及以丙烯腈为原料合成和制造上述物质的企业均是丙烯腈污染的主要来源。腈纶纤维燃烧时可释放也丙烯腈。
丙烯腈在水中是不稳定的,水中浓度1mg/L以下时不影响生物降解。当水中丙烯腈的浓度为10mg/L时,经过一昼夜剩下46%,二昼夜只剩下19%,四昼夜剩下5%,六昼夜只剩下3.6%当水中丙烯脯的浓度为75mg/L时,经过二昼夜剩下30.5%,六昼夜时为起初数量的4.5%。空气中嗅觉阈值浓度为1.7~23ppm,水中嗅觉阈值浓度为0.0031~50.4mg/L。
危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇明火、高热易燃烧,并放出有毒气体。与氧化剂、强酸、强碱、胺类、溴反应剧烈。在火场高温下,能发生聚合放热,使容器破裂。
燃烧(分解)产物:一氧化碳、二氧化碳、氧化氮、氰化氢。
3.现场应急监测方法:
快速检测管法;便携式气相色谱法《突发性环境污染事故应急监测与处理处置技术》万本太主编
直接进水样气相色谱法;气体检测管法
气体速测管(德国德尔格公司产品)
4.实验室监测方法:
| 监测方法 | 来源 | 类别 |
| 气相色谱法 | HJ/T37-1999 | 固定污染源排气 |
| 吡啶-苯胺比色法 | 《空气中有害物质的测定方法》(第二版),杭士平编 | 空气 |
| 纳氏试剂比色法 | 《化工企业空气中有害物质测定方法》化学工业出版社 | 化工企业空气 |
| 纳氏试剂比色法 | 《水质分析大全》张宏陶等编 | 水质 |
| 气相色谱法 | 《固体废弃物试验与分析评价手册》中国环境监测总站等译 | 固体废弃物 |
5.环境标准:
| 中国 (TJ36-79) | 车间空气中有害物质的最高容许浓度 | 2mg/m 3 [皮] |
| 中国 (TJ36-79) | 居住区大气中有害物质的最高容许浓度 | 0.05mg/m 3 (日均值) |
| 中国(GB16297-1996) | 大气污染物综合排放标准 | 最高允许排放浓度:22mg/m 3 (表2);26mg/m 3 (表1) 最高允许排放速率(kg/h):二级0.77~16(表2);0.91~19(表1);三级1.2~25(表2);1.4~29(表1) 无组织排放监控浓度限值(mg/m 3 ):0.60(表2);0.75(表1) |
| 中国(待颁布) | 饮用水源中有害物质的最高容许浓度 | 2.0mg/L |
| 中国(GB11607-89) | 渔业水质标准 | 0.5mg/L |
| 中国(GHZB1-1999) | 地表水环境质量标准(I、II、III类水域特定值) | 0.000058mg/L |
| 中国(GB8978-1996) | 污水综合排放标准 | 一级:2.0mg/L 二级:5.0mg/L 三级:5.0mg/L |
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用活性炭或其它惰性材料吸收。也可用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。喷雾状水冷却和稀释蒸气、保护现场人员、把泄漏物稀释成不燃物。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。废弃物处置方法:焚烧法,焚烧炉要有后燃烧室,焚烧炉排出的氮氧化物通过洗涤器除去。化学法,用乙醇氢氧化钠处理,将其产物同大量水一起排入下水道。另外,从废水中回收丙烯腈也是一种可考虑的处理办法。
二、防护措施
呼吸系统防护:可能接触毒物时,必须佩戴过滤式防毒面具(全面罩)。紧急事态抢救或撤离时,佩戴空气呼吸器。
眼睛防护:呼吸系统防护中已作防护。
身体防护:穿连衣式胶布防毒衣。
手防护:戴橡胶手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,彻底清洗。单独存放被毒物污染的衣服,洗后备用。车间应配备急救设备及药品。作业人员应学会自救互救。
三、急救措施
皮肤接触:立即脱去被污染的衣着,用流动清水或5%硫代硫酸钠溶液彻底冲洗至少20分钟。就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。呼吸心跳停止时,立即进行人工呼吸(勿用口对口)和胸外心脏按压术。给吸入亚硝酸异戊酯,就医。
食入:饮足量温水,催吐,用1:5000高锰酸钾或5%硫代硫酸钠溶液洗胃。就医。
灭火方法:消防人员必须佩戴过滤式防毒面具(全面罩)或隔离式呼吸器、穿全身防火防毒服,在上风处灭火。灭火剂:二氧化碳、干粉、砂土。用水灭火无效,但须用水保持火场容器冷却。
制备方法与用途
丙烯腈的生产方法主要有以下几种:
-
氰乙醇法:该方法以环氧乙烷和氢氰酸为原料,在水和三甲胺的存在下反应生成氰乙醇,然后在碳酸镁催化剂的作用下脱水制得丙烯腈。这种方法生产的丙烯腈纯度较高,但氢氰酸的毒性大且成本高。
-
乙炔法:该方法以乙炔、氢氰酸为原料,在氯化亚铜-氯化钾-氯化钠稀盐酸溶液催化剂作用下反应生成丙烯腈。此法制备过程简单,收率良好,但产物精制较难,毒性大,且原料成本较高。
-
丙烯氨氧化法:这是目前最有工业生产价值的生产方法。以丙烯、氨气和空气中的氧气为原料,在硅胶作载体的磷钼铋系或锑铁系催化剂作用下反应生成丙烯腈。该反应的单程收率可达75%,副产品有乙腈、氢氰酸和硫铵。
丙烯腈是一种易燃液体,具有高毒性。其急性毒性口服大鼠LD50为78毫克/公斤;小鼠27毫克/公斤。眼睛接触100毫克可引起中度刺激;皮肤接触50毫克可引起重度刺激。
在储存运输过程中需要注意以下几点:
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-丁烯腈 but-2-enenitrile 4786-20-3 C4H5N 67.0904 —— (Z)-3-aminoprop-2-enenitrile 24532-82-9 C3H4N2 68.0782 甲基丙烯腈 methacrylonitrile 126-98-7 C4H5N 67.0904 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (Z)-丁-2-烯二腈 maleonitrile 928-53-0 C4H2N2 78.0733 反丁烯二腈 1,2-Dicyanoethylene 764-42-1 C4H2N2 78.0733 —— trans-3-Chlor-acrylnitril —— C3H2ClN 87.5086 甲基丙烯腈 methacrylonitrile 126-98-7 C4H5N 67.0904
反应信息
-
作为反应物:参考文献:名称:Synthesis and physicochemical properties of chelating sorbents containing functional groups of N-aryl-3-aminopropionic acids摘要:合成了两种不同类型的螯合吸附剂,它们在聚合物基质中引入了亚氨基二丙酸基团:一种是基于线性聚苯乙烯的羧乙基氨基聚苯乙烯(吸附剂1),另一种是基于苯乙烯和二乙烯基苯共聚物的羧乙基氨基甲基聚苯乙烯(吸附剂2)。测定了吸附剂的功能基团的电离常数和浓度(对氢离子的交换容量)。这些吸附剂对铜(II)离子表现出高选择性,从氨-醋酸盐缓冲溶液中吸附铜(II)离子的最大值位于pH 5.0–7.5范围内。对于连续搅拌的铜(II)溶液与吸附剂系统,达到交换平衡所需的时间分别为3.5小时和2小时(吸附剂1和吸附剂2)。对铜(II)离子的交换容量分别为2.54和0.10 mmol g^-1。DOI:10.1007/s11172-006-0339-3
-
作为产物:参考文献:名称:钼酸铋催化剂上丙烯醛氨氧化为丙烯腈摘要:目前的工作涉及使用中孔钼酸铋催化剂将绿色起源的丙烯醛转化为丙烯腈的潜在重要过程。通过N 2物理吸附,X射线衍射和在各种条件下,不同温度,接触时间和反应物摩尔比下的催化试验,表征氨氧化催化剂。结果表明,催化活性与比表面积成比例,这取决于钼酸铋相和气体进料中氧气的浓度。催化剂的选择性仅取决于反应温度。在350–400°C下获得的ACN选择性为100%,在450°C下降至97%。DOI:10.1016/j.apcata.2016.03.030
-
作为试剂:描述:硫代乳酸 在 tetraethylammonium chlorochromate(VI) 、 丙烯腈 作用下, 以 二甲基亚砜 为溶剂, 生成 2,2'-dithiobispropanoic acid参考文献:名称:Swami, Preeti; Vadera; Prasadrao, Journal of the Indian Chemical Society, 2009, vol. 86, # 12, p. 1320 - 1324摘要:DOI:
文献信息
-
Nickel(II) N‐Heterocyclic Carbene Complexes: Versatile Catalysts for C–C, C–S and C–N Coupling Reactions作者:Lourdes Benítez Junquera、Francys E. Fernández、M. Carmen Puerta、Pedro ValergaDOI:10.1002/ejic.201700057日期:2017.5.18A variety of Ni(II) complexes with a wide range of electronic and steric properties, bearing picolyl-imidazolidene ligands (a-g) and Cp (2a-f) or Cp* (3a,c,g) groups, have been synthesised and characterised using NMR and single crystal X-ray crystallography. The complexes have been used as precatalysts for a wide range of catalytic transformations most likely involving a Ni0/NiII catalytic cycle. In
-
Discovery of triazolo [1,5-a] pyridine derivatives as novel JAK1/2 inhibitors作者:Kuan Lu、Weibin Wu、Cunlong Zhang、Zijian Liu、Boren Xiao、Zigao Yuan、Anqi Li、Dawei Chen、Xin Zhai、Yuyang JiangDOI:10.1016/j.bmcl.2020.127225日期:2020.7demonstrated efficacy in rheumatoid arthritis, inflammatory bowel disease, and psoriasis with the approval of several drugs. Aiming to develop potent JAK1/2 inhibitors, two series of triazolo [1,5-a] pyridine derivatives were designed and synthesized by various strategies. The pharmacological results identified the optimized compounds J-4 and J-6, which exerted high potency against JAK1/2, and selectivity
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOCAD JOINT STOCK CO公开号:WO2018092047A1公开(公告)日:2018-05-24The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.本发明涉及一种新的化合物,其化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为CH2,NH,O或化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[化学式II]每个R7,R8,R9,R10独立地为乙烯基,甲基乙炔基;Hal为CI,Br,I,F,具有布鲁顿酪氨酸激酶(Btk)抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。
-
Synthesis and biological activity of substituted 2,4-diaminopyrimidines that inhibit Bacillus anthracis作者:Baskar Nammalwar、Richard A. Bunce、K. Darrell Berlin、Christina R. Bourne、Philip C. Bourne、Esther W. Barrow、William W. BarrowDOI:10.1016/j.ejmech.2012.05.018日期:2012.8the lowest MICs with values of 0.5 μg/mL and 0.375–1.5 μg/mL, respectively. It is likely that the S isomers of 1 will bind the substrate-binding pocket of dihydrofolate reductase (DHFR) as in B. anthracis was found for (S)-1a. The final step in the convergent synthesis of target systems 1 from (±)-1-(1-substituted-2(1H)-phthalazinyl)-2-propen-1-ones 6 with 2,4-diamino-5-(5-iodo-3,4-dimethoxybenzyl)pyrimidine制备了一系列取代的 2,4-二氨基嘧啶1 ,并使用先前报道的 (±)-3-5-[(2,4-二氨基-5-嘧啶基)甲基]-2,3 评估了其抗炭疽杆菌的活性-二甲氧基苯基}-1-(1-丙基-2( 1H )-酞嗪基)-2-丙烯-1-酮( 1a ),最低抑菌浓度(MIC)值为1-3μg/mL,作为标准。在目前的工作中,相应的异丁烯基 ( 1e ) 和苯基 ( 1h ) 衍生物在最低 MIC 方面表现出最显着的活性,分别为 0.5 μg/mL 和 0.375–1.5 μg/mL。 1的S异构体很可能会结合二氢叶酸还原酶 (DHFR) 的底物结合袋,就像在炭疽芽孢杆菌中发现的 ( S ) -1a一样。由 (±)-1-(1-取代-2(1 H )-酞嗪基)-2-丙烯-1-酮6与 2,4-二氨基-5-( 收敛合成目标系统1的最后一步5-碘-3,4-二甲氧基苯甲基)嘧啶( 13 )是在密封管条件下通过新型Heck偶联反应完成的。
-
Melanocortin-4 receptor binding compounds and methods of use thereof申请人:Millennium Pharmaceuticals, Inc.公开号:US20040082779A1公开(公告)日:2004-04-29Provided are MC4-R binding compounds of the formula XVII: 1 wherein L 2 is a linker group, and P 1 , P 2 , P 3 , P 4 , Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , t, s, and R are as described in the specification. Methods of using the compounds to treat MC4-R associated disorders, such as disorders associated with weight loss, are also provided.
表征谱图
-
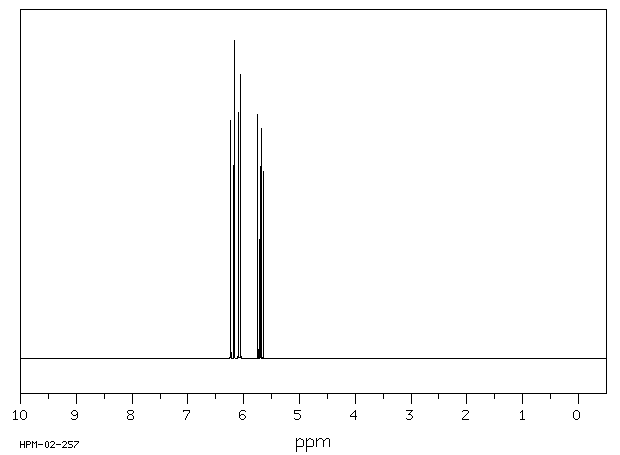
氢谱1HNMR
-
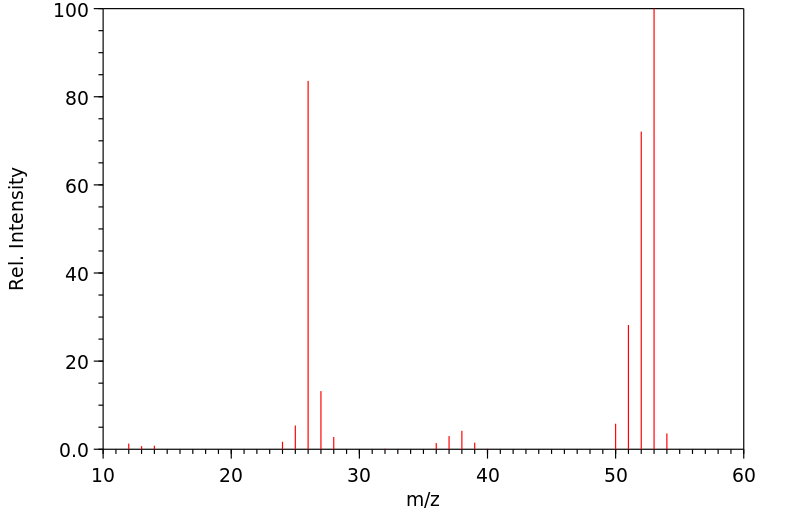
质谱MS
-
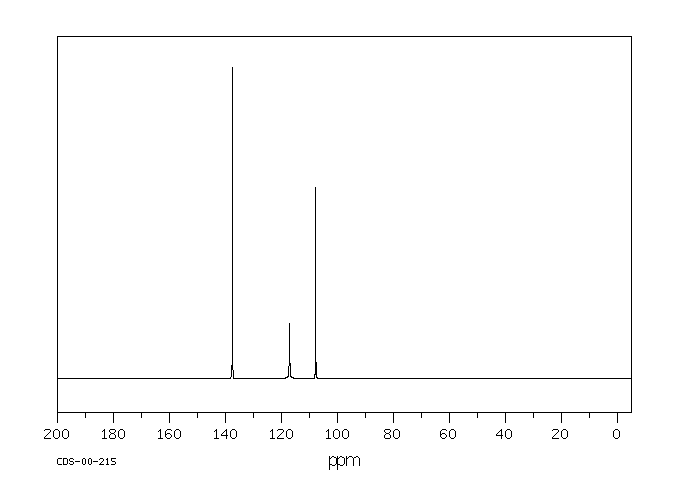
碳谱13CNMR
-
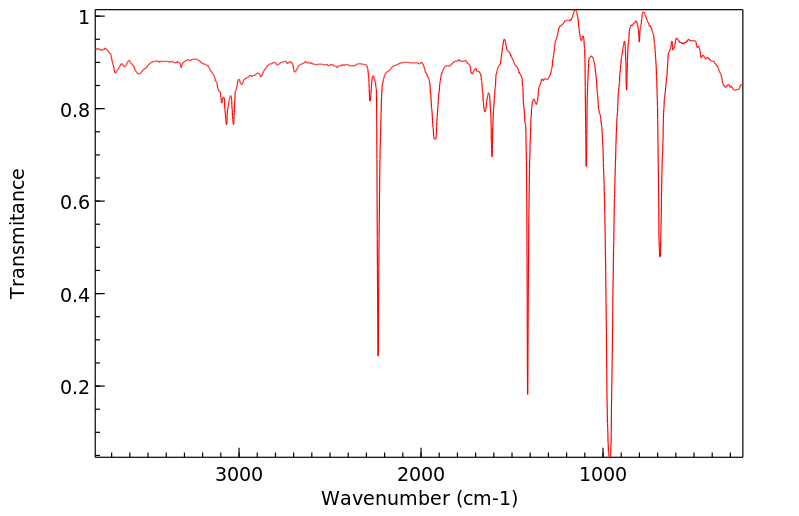
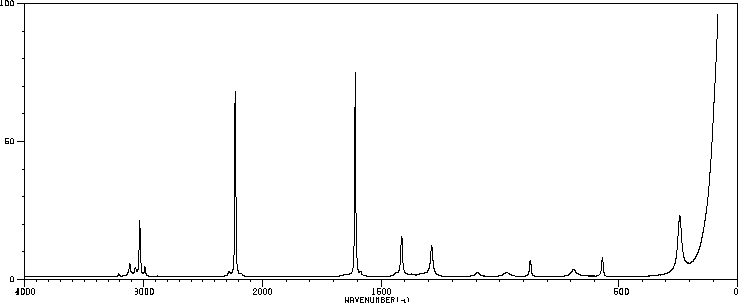
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息