对氯扁桃酸 | 492-86-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:114-118 °C
-
沸点:266.91°C (rough estimate)
-
密度:1.3245 (rough estimate)
-
碰撞截面:135.63 Ų [M-H]-
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2918199090
-
危险品运输编号:OTH
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封保存,并置于通风、干燥的环境中。
SDS
| Name: | 4-Chloromandelic acid 98% (titr.) Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 492-86-4 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 492-86-4 | 4-Chloromandelic acid, 98%(Titr.) | 98% | 207-764-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Use with adequate ventilation. Avoid breathing dust, vapor, mist, or gas.
Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Good general ventilation should be sufficient to control airborne levels.
Exposure Limits CAS# 492-86-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 115 - 121 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H7ClO3
Molecular Weight: 186.59
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidants.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 492-86-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Chloromandelic acid, 98%(Titr.) - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 492-86-4: No information available.
Canada
CAS# 492-86-4 is listed on Canada's NDSL List.
CAS# 492-86-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 492-86-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
白色或浅黄色针状结晶。熔点为119-122℃。易溶于醇和醚,并可溶解于热苯中;尚能溶于水,微溶于苯和二硫化碳。
用途
用作医药中间体。
生产方法
通过氯苯与乙酐反应生成对氯苯乙酮,再经溴化、水解及酸化而得。具体步骤如下:首先将氯苯、二硫化碳和无水三氯化铝混合,在搅拌下滴加乙酐并回流2小时后回收二硫化碳,然后倒入冰冷的稀盐酸中提取苯层。接着依次用水、10%氢氧化钠溶液洗涤,并干燥,通过减压蒸馏得到4-氯苯乙酮。随后在36-40℃下滴加溴素,再经过水洗和亚硫酸氢钠洗涤,最终用水洗后放置以获得4-ω,ω'-二溴苯乙酮结晶。将此结晶加入氢氧化钠液中进行水解,最后用盐酸中和至强酸性,经乙醚提取并干燥,以苯为溶剂结晶即得成品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对氯苯乙酸 4-chlorophenylacetic Acid 1878-66-6 C8H7ClO2 170.595 对氯苯乙酸乙酯 4-chloro-benzeneacetic acid, ethyl ester 14062-24-9 C10H11ClO2 198.649 —— 4-chloromandelonitrile 13312-83-9 C8H6ClNO 167.595 (4-氯苯基)乙醛酸 4-chlorophenylglyoxylic acid 7099-88-9 C8H5ClO3 184.579 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (R)-2-(4-氯苯基)-2-羟基乙酸 (R)-4-chloromandelic acid 32189-36-9 C8H7ClO3 186.595 L-对氯扁桃酸{S-(+)-对氯扁桃酸、S-(+)-4-氯扁桃酸} (S)-4-chloromandelic acid 76496-63-4 C8H7ClO3 186.595 —— methyl 4-chloromandelate —— C9H9ClO3 200.622 (4-氯苯基)羟基乙酸甲酯 methyl (R)-(-)-4-chloromandelate 32174-34-8 C9H9ClO3 200.622 —— 2-(4-chlorophenyl)-2-methoxyacetic acid 4674-24-2 C9H9ClO3 200.622 2-(4-氯苯基)-2-羟基乙酸乙酯 ethyl 2-(4-chlorophenyl)-2-hydroxyacetate 13511-29-0 C10H11ClO3 214.649 —— ethyl (R)-2-hydroxy-2-(4-chlorophenyl)acetate —— C10H11ClO3 214.649 D-扁桃酸 (R)-Mandelic Acid 611-71-2 C8H8O3 152.15 1-(4-氯苯基)乙烷-1,2-二醇 1-(p-chlorophenyl)-1,2-ethanediol 7477-64-7 C8H9ClO2 172.611 2-(4-氯苯基)-2-甲氧基乙酸甲酯 methyl 2-(4-chlorophenyl)-2-methoxyacetate 10399-10-7 C10H11ClO3 214.649 —— 2-(4-chlorophenyl)-2-(prop-2-yn-1-yloxy)acetic acid 655223-09-9 C11H9ClO3 224.644 2-(4-氯苯基)-2-羟基乙酰胺 2-(4-chlorophenyl)-2-hydroxyacetamide 18584-27-5 C8H8ClNO2 185.61 —— (S)-2-(4-chlorophenyl)-2-hydroxyacetamide 144664-11-9 C8H8ClNO2 185.61 —— (4-chloro-phenyl)-prop-2-ynyloxy-acetic acid methyl ester 663918-51-2 C12H11ClO3 238.671 a-羟基-[1,1-联苯]-4-乙酸 4-phenylmandelic acid 450-52-2 C14H12O3 228.247 —— methyl 2-acetoxy-2-(3-bromophenyl)acetate —— C11H11ClO4 242.659 —— 4-chloro-α-hydroxyphenylacetyl hydrazine 13312-93-1 C8H9ClN2O2 200.625 (4-氯苯基)乙醛酸 4-chlorophenylglyoxylic acid 7099-88-9 C8H5ClO3 184.579 —— 2-(4-chlorophenyl)-2-mercaptoacetic acid 114898-41-8 C8H7ClO2S 202.661 (R)-4-氯苯甘氨酸 (R)-2-amino-2-(4-chlorophenyl)acetic acid 43189-37-3 C8H8ClNO2 185.61 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:氯化亚胺氯化物对不对称邻位二醇的选择性单氯化摘要:已发现氯化亚胺氯化物可促进不对称邻位二醇的高效和选择性单氯化。Vilsmeier试剂,即(氯亚甲基)二甲基亚胺氯化物,能够对具有一个仲苄基羟基和一个伯脂族羟基的1,2-和1,3-二醇进行高反应性和区域选择性氯化,从而生成相应的1,2-和1 ,3-氯代醇。Viehe盐(α,α-二氯亚胺盐)显示出出色的反应性和邻邻二醇的良好选择性,可以通过环状中间体就地生成相应的氯醇氨基甲酸酯。氯化方案可耐受各种官能团,包括卤素,萘环,硝基和氰基。此外,在该氯化反应过程中可以保持手性二醇的光学纯度。DOI:10.1016/j.tet.2020.131114
-
作为产物:描述:2-(4-chlorophenyl)-N,2-dihydroxyacetamide 在 sodium hydroxide 作用下, 以 乙腈 为溶剂, 反应 3.0h, 以86%的产率得到对氯扁桃酸参考文献:名称:异羟肟酸的简单合成及其转化为α-羟基和α-氨基酸摘要:在羟基胺衍生物的存在下,LiBr或Li 2 NiBr 4对宝石-二氰基环氧化合物的亲核开环会导致新的α-卤代异羟肟酸。这些化合物已经以良好的产率用于合成α-官能化的异羟肟酸,α-羟基和α-氨基酸。DOI:10.1016/s0040-4039(00)00228-8
文献信息
-
[EN] 2,4-DIAMINOQUINAZOLINES FOR SPINAL MUSCULAR ATROPHY<br/>[FR] 2,4-DIAMINOQUINAZOLINES UTILES POUR LE TRAITEMENT D'UNE ATROPHIE MUSCULAIRE SPINALE申请人:DECODE CHEMISTRY INC公开号:WO2005123724A1公开(公告)日:2005-12-292,4-Diaminoquinazolines of formulae I-IV and VI (I, II, III, IV and VI) are useful for treating spinal muscular atrophy (SMA).2,4-二氨基喹唑啉的化学式I-IV和VI(I,II,III,IV和VI)可用于治疗脊髓性肌萎缩症(SMA)。
-
Oxidation of α-Hydroxy Acids with Quinolinium Dichromate - A Kinetic Study作者:Kailasa Aruna、Prerepa Manikyamba、Embar Venkatachari SundaramDOI:10.1135/cccc19931624日期:——
Oxidation of lactic acid, α-hydroxyphenyllacetic acid and its 4-chloro derivative with quinolinium dichromate (QDC) in 30% (v/v) aqueous acetic acid at 303 K are first order in QDC and first-order in hydroxy acids. The reactions are acid-catalyzed and a medium of low dielectric constant favours the oxidation. The products are the corresponding aldehydes. Thermodynamic parameters are evaluated and a mechanism involving a C-C bond cleavage is proposed.
-
Carboxylation with CO<sub>2</sub> via Brook Rearrangement: Preparation of α-Hydroxy Acid Derivatives作者:Tsuyoshi Mita、Yuki Higuchi、Yoshihiro SatoDOI:10.1021/ol403099f日期:2014.1.3rearrangement. A variety of α-substituents including aryl, alkenyl, and alkyl groups were tolerated to afford α-hydroxy acids in moderate-to-high yields. One-pot synthesis from aldehydes using PhMe2SiLi and CO2 was also possible, providing α-hydroxy acids without the isolation of an α-hydroxy silane.
-
Kinetic resolution of mandelate esters via stereoselective acylation catalyzed by lipase PS-30作者:Peiran Chen、Wenhong YangDOI:10.1016/j.tetlet.2014.02.095日期:2014.4By using lipase PS-30 as catalyst, the kinetic resolution of a series of racemic mandelate esters has been achieved via stereoselective acylation. The value of kinetic enantiomeric ratio (E) reached up to 197.5. Substituent effect is briefly discussed.
-
Synthesis of optically pure 4-aryl-2-hydroxytetronic acids申请人:The Ohio State University Research Foundation公开号:US05399721A1公开(公告)日:1995-03-21The present invention relates to a method for synthesis of optically pure stereogenically labile 4-aryl-2-hydroxytetronic acids from an optically pure aldehyde. The invention further relates to the use of such optically pure compounds as potent inhibitors of platelet aggregation by working at the level of cyclooxygenase. the invention further relates to the pharmaceutical use of such compounds in the treatment of coronary artery diseases, especially in the treatment and/or prevention of atherosclerosis.
表征谱图
-
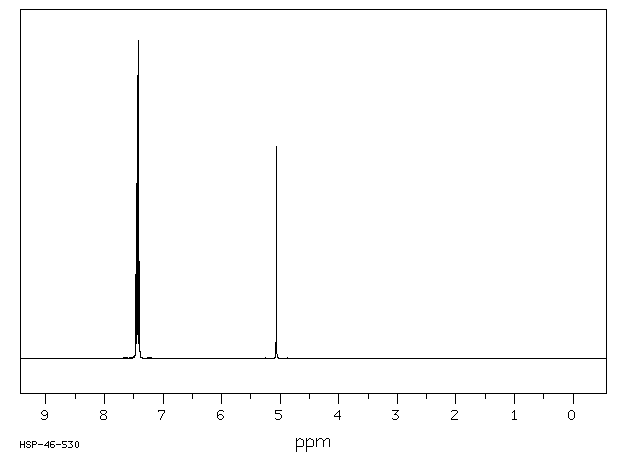
氢谱1HNMR
-
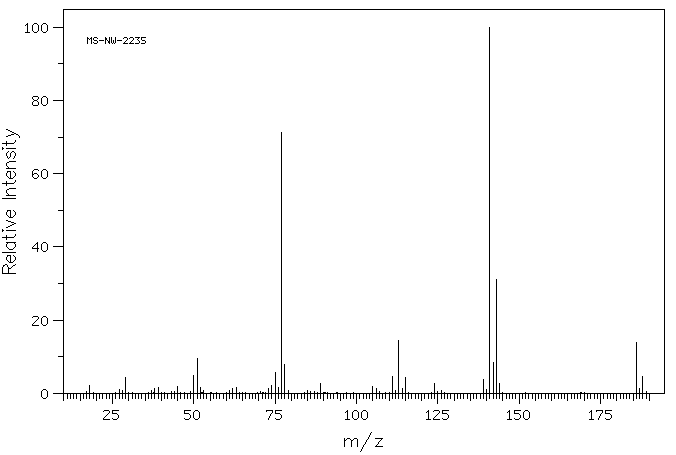
质谱MS
-
碳谱13CNMR
-
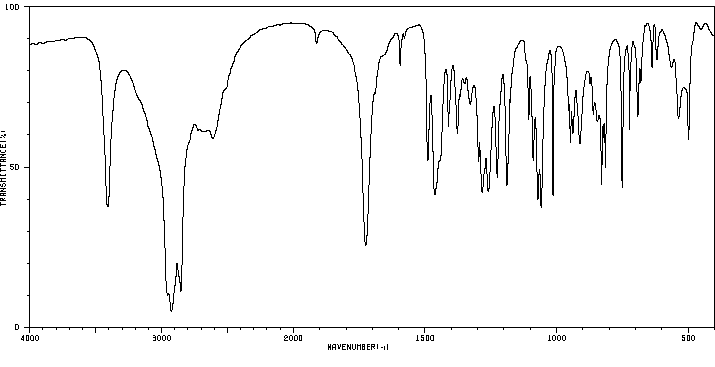
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息