2-噻酚乙酯乙酸乙酯 | 4075-58-5
中文名称
2-噻酚乙酯乙酸乙酯
中文别名
噻吩-2-乙醛酸乙酯
英文名称
ethyl 2-thienyl glyoxylate
英文别名
thienylglycoxylate dethyle;ethyl 2-oxo-2-(thiophen-2-yl)acetate;Ethyl thiophene-2-glyoxylate;ethyl 2-oxo-2-thiophen-2-ylacetate
CAS
4075-58-5
化学式
C8H8O3S
mdl
MFCD00015538
分子量
184.216
InChiKey
GHOVLEQTRNXASK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:102-110 °C (0.8 mmHg)
-
密度:1.25
-
闪点:>110°C
-
溶解度:难溶于水
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:71.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | 2-Thiophene glyoxylic acid ethyl ester 96% Material Safety Data Sheet |
| Synonym: | 1-Oxo-2-thiopheneacetic acid ethyl ester; Ethyl 1-oxo-2-thiopheneacetate |
| CAS: | 4075-58-5 |
Synonym:1-Oxo-2-thiopheneacetic acid ethyl ester; Ethyl 1-oxo-2-thiopheneacetate
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4075-58-5 | 2-Thiophene glyoxylic acid ethyl ester | 96 | 223-793-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
Causes skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
Causes gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Wash clothing before reuse.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4075-58-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 102.0 - 110.0 deg C @ .80mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 230 deg C (> 446.00 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.2570g/cm3
Molecular Formula: C8H8O3S
Molecular Weight: 184.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4075-58-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Thiophene glyoxylic acid ethyl ester - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 4075-58-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4075-58-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4075-58-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
2-噻酚乙酯乙酸乙酯可用作医药合成中间体,如之别IR吸收剂、有机半导体材料,尤其适用于有机光伏器件和光电二极管或含有二极管和/或有机场效应晶体管的器件。它在有机溶剂中具有优异的溶解性和成膜性质。
制备将9.77g AlCl3悬浮于50ml二氯甲烷中,然后在-20℃和氮气保护下滴加5g乙基乙二酰氯酯(ethylchlorooxoacetate)。接着,在-20℃条件下并搅拌的情况下滴加6.09g 2-噻吩。搅拌1小时后,将反应混合物倾倒入冰-水中,并使用乙酸乙酯进行萃取以分离化合物2-噻酚乙酯乙酸乙酯。最后通过柱层析纯化产物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-噻吩乙醛酸 2-thiopheneglyoxylic acid 4075-59-6 C6H4O3S 156.162 2-噻吩乙酸乙酯 ethyl thiophen-2-ylacetate 57382-97-5 C8H10O2S 170.232 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— isopropyl-2-oxo-2-(thiophen-2-yl)acetate —— C9H10O3S 198.243 2-噻吩乙醛酸 2-thiopheneglyoxylic acid 4075-59-6 C6H4O3S 156.162 —— (1-methyl-4-piperidyl)methyl-2-oxo-2-(2-thienyl)acetate —— C13H17NO3S 267.349 2-噻吩乙酸乙酯 ethyl thiophen-2-ylacetate 57382-97-5 C8H10O2S 170.232 —— oxothien-2-yl-acetic acid 1-azabicyclo[2.2.2]oct-3(R)-yl ester 320348-21-8 C13H15NO3S 265.333 —— (R)-ethyl 2-hydroxy-2-(thiophen-2-yl)acetate —— C8H10O3S 186.232 —— (R)-ethyl 2-hydroxy-2-(thiophen-2-yl)acetate —— C8H10O3S 186.232 2-羟基-2-(噻吩-2-基)乙酸乙酯 α-hydroxy-α-(thiophen-2-yl) ethyl acetate 62323-55-1 C8H10O3S 186.232
反应信息
-
作为反应物:描述:2-噻酚乙酯乙酸乙酯 在 sodium hydroxide 作用下, 生成 2-噻吩乙醛酸参考文献:名称:对映和化学选择性布朗斯台德酸/ Mg(nBu)2催化儿茶酚硼烷还原α-酮酯。摘要:已开发出第一对映体和化学选择性布朗斯台德酸催化的儿茶酚硼烷还原α-酮酯。α-羟基酯是在温和的反应条件下以实际上定量的产率和优异的对映选择性而获得的。稍作修饰,即可得到两种对映体,而不会损失任何选择性。DOI:10.1039/c4cc00427b
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 lithium diisopropyl amide 作用下, 生成 2-噻酚乙酯乙酸乙酯参考文献:名称:将格氏试剂添加到1-(N-(烷氧基草酰)-N-甲基氨基)-3-甲基咪唑鎓盐中:合成α-酮酯的通用方法。摘要:DOI:10.1021/jo961329z
-
作为试剂:描述:参考文献:名称:Thiophenyl triazol-3-one derivatives as smooth muscle relaxants摘要:本发明提供了一种新型的[1,2,4]三唑-3-酮衍生物,其具有一般式(I),其中:Q为,R1、R2、R3、R4、R5和R6如本文所定义,或其无毒药学上可接受的盐或溶剂,它们是平滑肌松弛剂,并且在治疗对平滑肌松弛有反应的疾病,如哮喘、肠易激综合征、男性勃起功能障碍和尿失禁方面有用。公开号:US06613786B2
文献信息
-
A new one step synthesis of maleimides by condensation of glyoxylate esters with acetamides作者:Margaret M. Faul、Leonard L. Winneroski、Christine A. KrumrichDOI:10.1016/s0040-4039(98)02594-5日期:1999.2Bisphenyl, Bisheteroaryl, indolylaryl and indolylcycloalkyl maleimides are prepared in one step and 67–99% yield by condensation of glyoxylate esters with acetamides using a 1.0 M solution of potassium tert-butoxide in THF. The mechanism of the reaction is discussed.
-
[EN] ALKALOID AMINOESTER DERIVATIVES AND MEDICINAL COMPOSITION THEREOF<br/>[FR] DÉRIVÉS AMINOESTER D'ALKALOÏDES ET COMPOSITION MÉDICINALE LES COMPRENANT申请人:CHIESI FARMA SPA公开号:WO2011160919A1公开(公告)日:2011-12-29The present invention relates to alkaloid aminoester derivatives acting as muscarinic receptor antagonists, processes for their preparation, compositions comprising them and therapeutic uses thereof.
-
ALKALOID AMINOESTER DERIVATIVES AND MEDICINAL COMPOSITION THEREOF申请人:AMARI Gabriele公开号:US20110311459A1公开(公告)日:2011-12-22The present invention relates to alkaloid aminoester compounds which act as muscarinic receptor antagonists, processes for the preparation of such a compound, compositions which contain such a compound, and therapeutic uses of such a compound.
-
A Phosphetane Catalyzes Deoxygenative Condensation of α-Keto Esters and Carboxylic Acids via P<sup>III</sup>/P<sup>V</sup>═O Redox Cycling作者:Wei Zhao、Patrick K. Yan、Alexander T. RadosevichDOI:10.1021/ja511889y日期:2015.1.21A small-ring phosphacycle is found to catalyze the deoxygenative condensation of α-keto esters and carboxylic acids. The reaction provides a chemoselective catalytic synthesis of α-acyloxy ester products with good functional group compatibility. Based on both stoichiometric and catalytic mechanistic experiments, the reaction is proposed to proceed via catalytic P(III)/P(V)═O cycling. The importance
-
Diastereo- and Enantioselective Synthesis of Fluorine Motifs with Two Contiguous Stereogenic Centers作者:Sudipta Ponra、Wangchuk Rabten、Jianping Yang、Haibo Wu、Sutthichat Kerdphon、Pher G. AnderssonDOI:10.1021/jacs.8b08778日期:2018.10.24motifs, in particular, chiral fluorine molecules with two contiguous stereogenic centers, has attracted much interest in research due to the limited number of methods available for their preparation. Herein, we report an atom-economical and highly stereoselective synthesis of chiral fluorine molecules with two contiguous stereogenic centers via azabicyclo iridium-oxazoline-phosphine-catalyzed hydrogenation
表征谱图
-
氢谱1HNMR
-
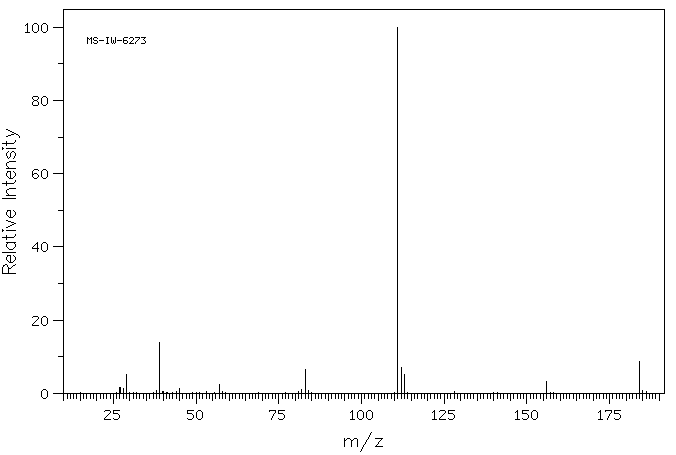
质谱MS
-
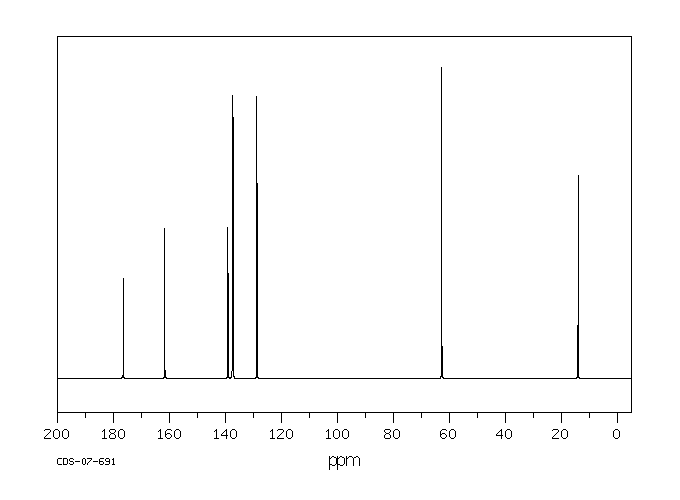
碳谱13CNMR
-
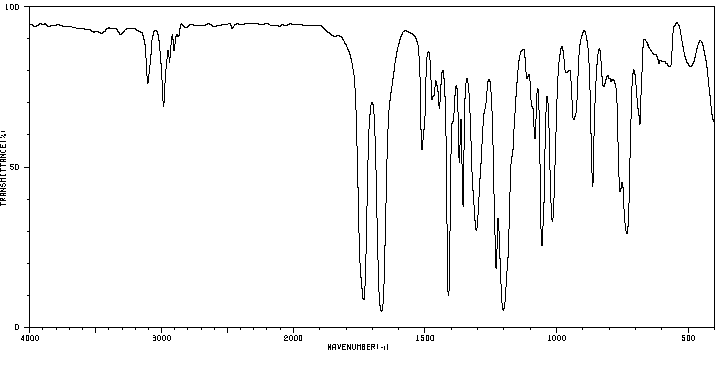
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷