2-噻酚基环丙基甲酮 | 6193-47-1
中文名称
2-噻酚基环丙基甲酮
中文别名
环丙基噻吩甲酮;环丙基2-噻吩基酮;环丙基-2-噻吩基甲酮
英文名称
cyclopropyl(2-thienyl)methanone
英文别名
cyclopropyl(thiophen-2-yl)methanone;cyclopropyl 2-thienyl ketone
CAS
6193-47-1
化学式
C8H8OS
mdl
MFCD00005427
分子量
152.217
InChiKey
PJDFNFSTSCAKPC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:120 °C / 3mmHg
-
密度:1.17 g/mL at 25 °C(lit.)
-
闪点:198 °F
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:10
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.375
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2934999090
-
储存条件:Dark Room
SDS
环丙基-2-噻吩基甲酮 修改号码:5
模块 1. 化学品
产品名称: Cyclopropyl 2-Thienyl Ketone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 环丙基-2-噻吩基甲酮
百分比: >95.0%(GC)
CAS编码: 6193-47-1
分子式: C8H8OS
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
环丙基-2-噻吩基甲酮 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 120 °C/0.4kPa
闪点: 92°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.18
环丙基-2-噻吩基甲酮 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
环丙基-2-噻吩基甲酮 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Cyclopropyl 2-Thienyl Ketone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 环丙基-2-噻吩基甲酮
百分比: >95.0%(GC)
CAS编码: 6193-47-1
分子式: C8H8OS
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
环丙基-2-噻吩基甲酮 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 120 °C/0.4kPa
闪点: 92°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.18
环丙基-2-噻吩基甲酮 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
环丙基-2-噻吩基甲酮 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯-1-(2-噻吩基)丁酮 4-chloro-1-(thien-2-yl)-1-butanone 43076-59-1 C8H9ClOS 188.678 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4-bromothiophen-2-yl)(cyclopropyl)methanone 1065185-71-8 C8H7BrOS 231.113 —— 4-bromo-2-(cyclopropylmethyl)thiophene 1065184-58-8 C8H9BrS 217.129
反应信息
-
作为反应物:描述:2-噻酚基环丙基甲酮 在 bis(1,5-cyclooctadiene)nickel (0) 、 potassium tert-butylate 、 1,3-双(2,6-二异丙基苯基)氯化咪唑鎓 作用下, 以 甲苯 为溶剂, 以88%的产率得到((1RS,3RS)-2-methylcyclopentane-1,3-diyl)bis(tiophen-2-ylmetanone)参考文献:名称:环丙基酮的二聚化和环丙基酮与作为五元环入口的烯酮的交叉反应摘要:观察到环丙基酮出人意料的二聚化,并且对反应途径的分析导致了环丙基酮和烯酮之间合成有用的交叉反应的发展,以提供密集官能化的环戊烷产物。DOI:10.1021/ja0602187
-
作为产物:描述:参考文献:名称:钯催化芳基环丙基酮的立体选择性开环反应摘要:在此,我们报道通过钯催化的单取代环丙基酮的开环可以有效且系统地获得α,β-不饱和酮。( E )-1-Arylbut-2-en-1-ones 通过 Pd(OAc) 2 /PCy 3催化体系由芳基环丙基酮立体选择性生成,收率为 23-89% 。该反应表现出立体选择性(仅发现E产物)并且适用于苯基和杂芳基环丙基酮。DOI:10.1039/d2ob00719c
文献信息
-
α-Lithiated Aryl Benzyl Ethers: Inhibition of [1,2]-Wittig Rearrangement and Application to the Synthesis of Benzo[<i>b</i>]furan Derivatives作者:Rocío Velasco、Claudia Feberero、Roberto SanzDOI:10.1021/acs.orglett.5b01964日期:2015.9.18low temperature selectively leads to α-lithiation of benzyl phenyl ether generating a stable organolithium, which can be efficiently trapped with a variety of selected electrophiles prior to suffering the expected [1,2]-Wittig rearrangement. In the case of (o-alkynyl)phenyl benzyl ethers, the intermediate α-aryloxyorganolithium undergoes an unexpected anti intramolecular carbolithiation reaction leading
-
Ruthenium-Catalyzed Reactions of 1-Cyclopropyl-2-propyn-1-ols with Anilines and Water via Allenylidene Intermediates: Selective Preparation of Tri- and Tetrasubstituted Conjugated Enynes作者:Yoshihiro Yamauchi、Gen Onodera、Ken Sakata、Masahiro Yuki、Yoshihiro Miyake、Sakae Uemura、Yoshiaki NishibayashiDOI:10.1021/ja0687926日期:2007.4.1Ruthenium-catalyzed efficient preparation of the conjugated enynes can be carried out in the reactions of 1-cyclopropyl-2-propyn-1-ols with nitrogen- and oxygen-centered nucleophiles such as anilines and water in the presence of a catalytic amount of sulfur-bridged diruthenium complexes. The use of such complexes as catalysts realizes the completely stereoselective preparation of tri- and tetrasubstituted
-
An efficient and transition metal free protocol for the transfer hydrogenation of ketones as a continuous flow process作者:Jörg Sedelmeier、Steven V. Ley、Ian R. BaxendaleDOI:10.1039/b821752a日期:——We report the efficient reduction of a selection of ketones to the corresponding secondary alcohols using only catalytic amounts of LiOtBu in iPrOH facilitated by using a continuous flow reactor.我们报告了一种高效的方法,通过在异丙醇中仅使用少量催化量的LiOtBu,并借助连续流动反应器,将一系列酮还原为相应的仲醇。
-
Palladium-catalysed direct regioselective C5-arylation of a thiophene bearing a cyclopropyl ketone group at C2作者:Anissa Beladhria、Aditya L. Gottumukkala、Chiraz Youssef、Charles Beromeo Bheeter、Hamed Ben Ammar、Ridha Ben Salem、Henri DoucetDOI:10.1016/j.catcom.2013.07.014日期:2013.11at C2 was successfully employed in palladium-catalysed direct arylation. The reaction proceeds regioselectively at C5 without decomposition of the cyclopropyl ketone substituent. These couplings were performed employing as little as 0.5 mol% of ligand-free Pd(OAc)2 catalyst with electron-deficient aryl bromides. A wide variety of functional groups on the aryl bromide such as nitrile, nitro, acetyl, formyl
-
[EN] A SOLVENT DRYING COMPOSITION AND PROCESSES THERFOR<br/>[FR] COMPOSITION DE SÉCHAGE PAR SOLVANT ET PROCESSUS ASSOCIÉS申请人:AQUAFORTUS TECH LIMITED公开号:WO2020204733A1公开(公告)日:2020-10-08The present disclosure relates to a solvent drying composition and processes therefor. The present disclosure more specifically relates to a solvent drying composition that in use releases water from a solvent mixture. The present disclosure also relates to a process for recovering a solvent drying composition, more specifically to a process for recovering a solvent drying composition used in an osmotic process.本公开涉及溶剂干燥组合物及其处理过程。具体而言,本公开涉及一种在使用中从溶剂混合物中释放水的溶剂干燥组合物。本公开还涉及一种用于回收溶剂干燥组合物的过程,更具体地说是用于回收在渗透过程中使用的溶剂干燥组合物的过程。
表征谱图
-
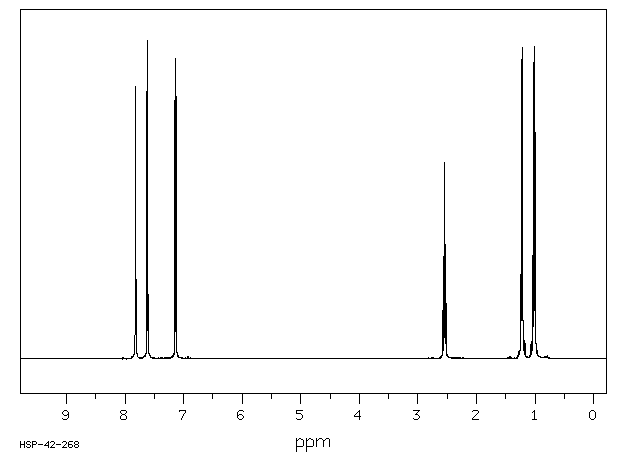
氢谱1HNMR
-
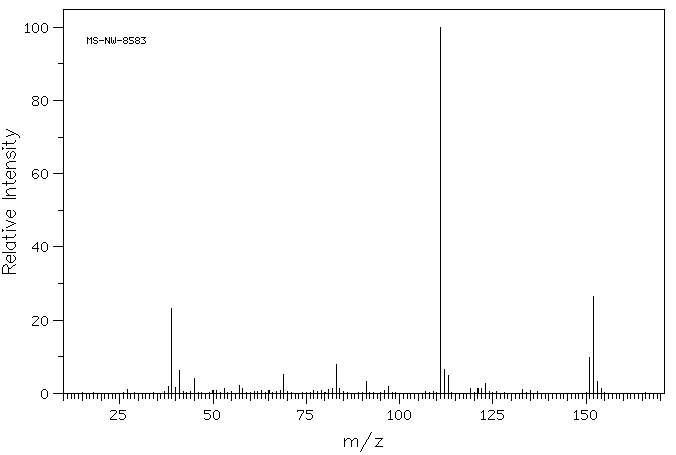
质谱MS
-
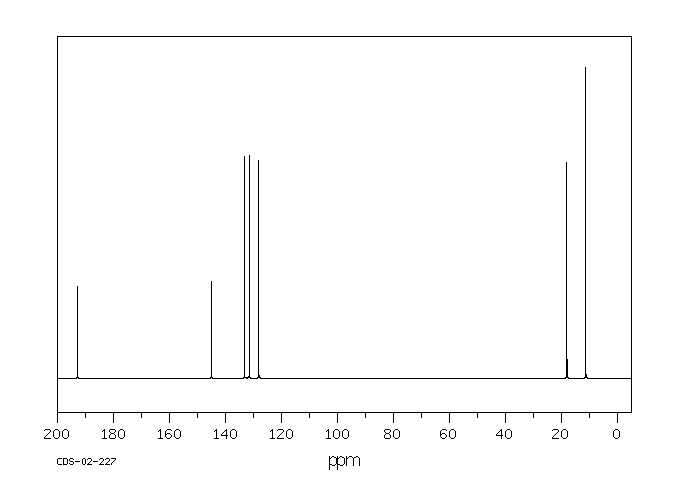
碳谱13CNMR
-
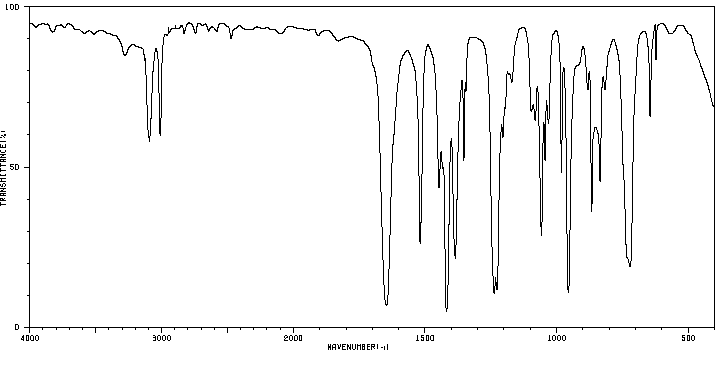
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷