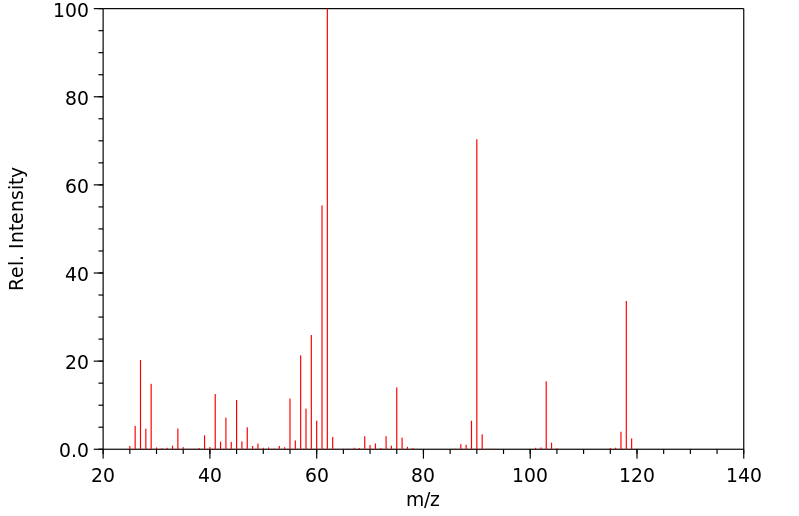
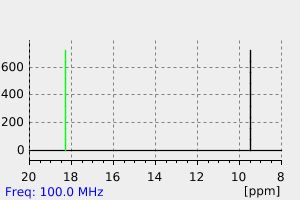
| Name: | Triethylphosphine 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 554-70-1 |
Section 1 - Chemical Product MSDS Name:Triethylphosphine 99% Material Safety Data Sheet
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS | CAS# | Chemical Name | content | EINECS# |
| 554-70-1 | Triethylphosphine | 99% | 209-068-8 |
Hazard Symbols: Not available.
Risk Phrases:
Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES Eyes: Not available.
Skin:
Not available.
Ingestion:
Not available.
Inhalation:
Not available.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES General Information:
Not available.
Extinguishing Media:
Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Not available.
Section 7 - HANDLING and STORAGE Handling:
Not available.
Storage:
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls:
Personal Protective Equipment Eyes: Not available.
Skin:
Not available.
Clothing:
Not available.
Respirators:
Not available.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Not available.
Appearance: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Vapor Density: Not available.
Evaporation Rate: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Decomposition Temperature: Not available.
Solubility: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: Not available.
Molecular Weight: Not available.
Section 10 - STABILITY AND REACTIVITY Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Not available.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION RTECS#:
CAS# 554-70-1: SZ3400000 LD50/LC50:
Not available.
Carcinogenicity:
Triethylphosphine - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION For further information, contact Fisher Scientific.
Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION CDG/CPL
IMO
Not regulated as a hazardous material.
IATA
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 554-70-1:
Canada
CAS# 554-70-1 is listed on Canada's DSL/NDSL List.
WHMIS: Not available.
CAS# 554-70-1 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 554-70-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION N/A