三苯基乙酸锡 | 900-95-8
中文名称
三苯基乙酸锡
中文别名
醋酸三苯基锡;三苯基锡醋酸盐;三苯基锡;三苯基醋酸锡;薯瘟锡
英文名称
triphenyltin acetate
英文别名
Triphenylzinnacetat;fentin acetate;triphenylstannyl acetate
CAS
900-95-8
化学式
C20H18O2Sn
mdl
——
分子量
409.072
InChiKey
WDQNIWFZKXZFAY-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118-122°C
-
沸点:60 °C
-
密度:1.55
-
溶解度:可溶于氯仿(少许)
-
物理描述:Solid
-
颜色/状态:Colorless crystals
-
蒸汽压力:1.4e-08 mm Hg at 140 °F (EPA, 1998)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与酸和氧化物接触。
-
自燃温度:IT IS NEITHER FLAMMABLE NOR AUTOIGNITIBLE.
-
分解:Unstable in acids & alkalis (22 °C), decomp half-time <3 hr (pH 5,7 or 9).
计算性质
-
辛醇/水分配系数(LogP):0.96
-
重原子数:23
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:40.1
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
INTACT (113)TIN-DIPHENYLTIN & (113)TIN-MONOPHENYLTIN WERE ONLY CMPD IDENTIFIED IN FECES OF GUINEA PIGS ADMIN (113)TIN-TRIPHENYLTIN ACETATE SUBCUTANEOUSLY.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Triphenyltin acetate, which is resistant to the in-vitro dealkylation system, /the microsome-soluble reduced nicotinamide adenine dinucleotide system containing cytochrome p450 dependent mono-oxygenase/ is metabolized, probably by some other mechanism to diphenyl- & monophenyltin, & inorganic tin.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A4;不可分类为人类致癌物。/有机锡化合物,作为Sn/
A4; Not classifiable as a human carcinogen. /Organic tin cmpd, as Sn/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别:三苯基锡化合物是四价锡的三苯基衍生物。它们是无色固体,具有很低的蒸汽压。它们具有亲脂性,在水中的溶解度很低。自20世纪60年代以来,三苯基锡化合物被广泛用作防污漆中的杀藻剂和杀软体动物剂。由于对牡蛎业产生的灾难性影响以及对水生生态系统的更广泛影响,许多国家对在防污漆中使用三有机锡进行了限制。人类暴露:没有关于职业暴露于三苯基锡化合物的数据。一些中毒案例报告描述了神经毒性效应,这些效应似乎持续存在。公众对三苯基锡化合物的暴露主要来自于摄入受污染的海产品。动物/植物研究:口服给予大鼠的三苯基锡化合物不易被吸收,主要随粪便排出,部分随尿液排出。它们被代谢为二苯基锡和一苯基锡,以及不可提取的结合残留物。吸收的三苯基锡化合物在肾脏和肝脏中积累到最大程度,其他器官中含量较少。三苯基锡化合物,通过皮肤给药可以以时间和剂量依赖性的方式透过皮肤。三苯基锡在各种动物种类中表现出多种健康效应,包括对免疫系统的影响、在接近母体毒性水平的生殖/发育效应、在内分泌器官中的增生/腺瘤、胸腺细胞的凋亡、肌质网细胞的钙释放和眼睛刺激。三苯基锡化合物对大鼠具有中等急性毒性。它们不是致癌的,但一些数据显示它们是促裂变剂。生殖和发育效应包括植入数量和活胎儿数量的减少(在兔子的灌胃研究中,每天每千克体重1.0毫克三苯基锡醋酸),在断奶大鼠的饮食中(每天每千克体重1.5毫克三苯基锡醋酸)减少窝大小/仔重和相对胸腺或脾重。三苯基锡化合物影响免疫系统。三苯基锡化合物在低浓度下对水生生物产生有害影响。由于造成雌性腹足类动物发育出雄性性器官的这种现象,三苯基锡被认为是内分泌干扰物。/三苯基锡化合物和三苯基锡醋酸/
IDENTIFICATION: Triphenyltin compounds are triphenyl derivatives of tetravalent tin. They are colorless solids with low vapor pressures. They are lipophilic and have low solubility in water. Triphenyltin compounds have been used extensively as algicides and molluscicides in antifouling paints since the 1960s. Use of triorganotins in antifouling paints has been restricted in many countries because of their catastrophic effects on the oyster industry and more general effects on the aquatic ecosystem. HUMAN EXPOSURE: There are no data concerning the occupational exposure to triphenyltin compounds. A few poisoning case reports does describe neurotoxic effecys, which appeared to persist. Exposure of the general public to triphenyltin compounds occurs mostly from ingestion of contaminated seafood. ANIMAL/PLANT STUDIES: Triphenyltin compounds given orally to rats are not readily absorbed and are excreted primarily in the feces and partly in the urine. They are metabolized to diphenyltin, and monophenyltin, and non-extractable bound residues. Absorbed triphenyltin compounds accumulate in the kidney and liver to the greatest extent, with smaller amounts in other organs. Triphenyltin compounds, applied dermally can penetrate through the skin in a time and dose dependent manner. Triphenyltin exerts a variety of health effects in various animal species, including effects on the immune system, reproductive/developmental effects at levels near those that are maternially toxic, hyperplasia/adenomas in endocrine organs, apoptosis in thymus cells, calcium release in sarcoplasmic reticulum cells, and eye irritation. Triphenyltin compounds are moderately acutely toxic to rats. They are not carcinogenic, but some data show that they are co-clastogenic. Reproductive and developmental effects include a decrease in the number of inplantations and live fetuses (at 1.0 mg triphenyltin acetate/kg body weight per day in a rabbit gavage study), reduction in litter size/pup weight and in relative thymus or spleen weightin the weanlings (at 1.5 mg triphenyltin acetate/kg body weight per day in the diet of a two generation reproduction study in rats). Triphenyltin compounds affect the immune system. Triphenyltin compounds exert deleterous effects on aquatic organisms at low concentrations. Triphenyltin is considered an endocrine disruptor, because of imposex, a phenomenon in which female gastropods develop male sex organs. /Triphenyltin cmpd & Triphenyltin acetate/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
职业暴露导致几起人类中毒事件,但未有死亡案例。严重头痛是一个常见症状,伴随的症状还包括恶心、呕吐和上腹痛,即使在呼吸道暴露的情况下也是如此。在几个受害者中发现了糖尿和高血糖,还有眩晕...两名农民喷洒后出现意识丧失。
Several human poisonings from occupational exposures have been described but no deaths. Severe headache is a common symptom, as are nausea, vomiting, and epigastric pain, even in respiratory exposures. Glycosuria and hyperglycemia were encountered in several victims, as was dizziness, ... loss of consciousness occurred in 2 farmers after spraying.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
肝脏损伤,在一例中不可逆转,已经在喷洒三苯基锡醋酸的人群中报告,尽管不能排除接触其他有毒化合物的可能性。一位患者在入院时,血液中锡含量为48微克/升,尿液中锡含量为113微克/升,主要症状为全身不适、头痛、眩晕和肝脏肿大,在喷洒了60%三苯基锡醋酸溶液后。入院九天后,他出现了弥漫性红斑,但三天后完全康复。
Liver damage, in one case irreversible, has been reported in people spraying triphenyltin acetate, although exposure to other toxic compounds cannot be excluded. General malaise, headache, dizziness, & enlarged liver were the main symptoms in a patient who had 48 ug tin/l in his blood & 113 ug tin/l in his urine at the time of admission to hospital, after spraying a 60% triphenyltin acetate solution. Nine days after admission he developed diffuse erythema, but completely recovered 3 days later.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
大多数因系统性影响而导致的意外中毒是由于职业暴露于三苯基锡醋酸。据报道,皮肤和吸入暴露后出现的系统性影响包括一般不适、胃痛、口干、视觉障碍和呼吸急促。在一些病例中发现了肝肿大和肝转氨酶活性升高。恢复通常是完全的,但在一个案例中,一名男子将一种农业化合物溅到手上和胸部,肝损伤持续了2年。
The majority of accidental poisonings involving systemic effects have been due to occupational exposure to triphenyltin acetate. Systemic effects reported to have followed both dermal & inhalation exposure include general malaise, gastric pain, dryness of the mouth, visual disturbances & shortness of breath. An enlarged liver & elevated levels of liver aminotransferase activity have been found in some cases. Recovery has generally been complete but in one case, where a man spilled an agricultural cmpd on his hands & chest, the liver damage persisted for 2 yr.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
基于对大鼠、小鼠和豚鼠的口服和腹腔给药,从胃肠道吸收较差,与腹膜吸收相比。
... /Based on oral & ip admin to rats, mice & guinea pigs/ absorption from the GI tract is poor compared with that from the peritoneum.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
从皮肤吸收较差。在给绵羊口服醋酸三苯基锡,剂量为每天10毫克,持续20天的情况下,在牛奶中发现了锡,平均浓度为0.0017 ppm。(113)锡以醋酸三苯基锡的形式存在,以及两种未识别的形式。停止给药17天后,浓度降至可检测限以下。在治疗期间,(113)锡在血液中的浓度为0.0029 ppm,在尿液中的浓度为0.0075 ppm。停止给药8天后,肝脏、肾脏、肺、胰腺、胆囊和大脑中的(113)锡含量高于其他器官,且在218天后肝脏中的水平仍在增加。在牛和绵羊中,三苯基锡主要通过粪便排出。三苯基锡迅速分布到所有组织,包括大鼠的大脑。在单次给药后30多天,仍可在大鼠和豚鼠的大脑中检测到。
Absorption from the skin is poor. During oral dosing of sheep for 20 days with fentin acetate at the rate of 10 mg/day, tin was found in milk at an avg concn of 0.0017 ppm. (113)Tin was present as fentin acetate & in 2 unidentified forms. Seventeen days after dosing was stopped, the concn had fallen to the limits of detectability. During treatment, the concn of (113)tin was 0.0029 ppm in the blood & 0.0075 ppm in the urine. Eight days after dosing was stopped, the liver, kidney, lung, pancreas, gall bladder, & brain contained higher concn of (113)tin than did other organs, & the level was still incr in the liver after 218 days. In cows & sheep, triphenyltin is excreted chiefly in the feces. Triphenyltin is rapidly distributed to all tissues, including the brain of rats. It can still be detected in the brain of rats & guinea pigs more than 30 days after a single dose.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
当被动物摄入时,可能是作为绿饲料上的残留物...它/会迅速随粪便排出体外...。少量吸收的部分通过尿液排出,部分分布到全身。
WHEN TAKEN IN BY ANIMALS, PROBABLY AS RESIDUE ON GREEN FODDER ... /IT/ WAS RAPIDLY EXCRETED WITH /FECES/. ... SMALL AMT ABSORBED WAS PARTLY EXCRETED IN URINE & PARTLY DISTRIBUTED THROUGHOUT WHOLE BODY.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
(113)锡-三苯基锡醋酸在豚鼠皮肤上的应用导致3.04%和7.97%的剂量通过皮肤被吸收,而在处理后的1天和2天,在应用区域发现了16.3%和12.4%。在大鼠皮下注射(113)锡-三苯基锡醋酸后,大量放射性物质在肝脏、肾脏和大脑中被发现,尽管吸收过程从注射部位缓慢进行。大约83%的剂量在20天内通过粪便排出,生物半衰期从豚鼠皮下注射(113)锡-三苯基锡醋酸估计为9.4天。在皮下给药后,粪便中仅发现的完整化合物是(113)锡-二苯基锡和(113)锡-单苯基锡。
CUTANEOUS APPLICATION OF (113)TIN-TRIPHENYLTIN ACETATE IN GUINEA PIGS RESULTED IN 3.04% & 7.97% OF DOSE BEING ABSORBED THROUGH SKIN, WHILE 16.3% & 12.4% WERE FOUND IN APPLICATION AREA 1 & 2 DAYS AFTER TREATMENT. RADIOACTIVITY WAS FOUND IN LIVER, KIDNEY & BRAIN IN LARGE AMT FOLLOWING SC INJECTION OF (113)TIN-TRIPHENYLTIN ACETATE, ALTHOUGH ABSORPTION PROCEEDED SLOWLY FROM INJECTION SITE. ABOUT 83% OF DOSE WAS ELIMINATED THROUGH FECES WITHIN 20 DAYS & BIOLOGICAL HALF TIME WAS EST AT 9.4 DAYS FROM SC INJECTION OF (113)TIN-TRIPHENYLTIN IN GUINEA PIGS. INTACT (113)TIN-DIPHENYLTIN & (113)TIN-MONOPHENYLTIN WERE ONLY CMPD IDENTIFIED IN FECES AFTER SC ADMIN.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T+,N
-
安全说明:S26,S28,S36/37/39,S45,S60,S61
-
危险类别码:R63,R40,R24/25,R26,R48/23,R50/53,R37/38,R41
-
危险品运输编号:3146
-
RTECS号:WH6650000
-
包装等级:III
-
危险类别:6.1
-
储存条件:请将容器密封保存,并存放在阴凉干燥处。
SDS
| 国标编号: | 61884 |
| CAS: | 900-95-8 |
| 中文名称: | 薯瘟锡 |
| 英文名称: | Fentin-acetate |
| 别 名: | 乙酸三苯基锡;乙酰氧基三苯基锡 |
| 分子式: | C 20 H 18 O 2 Sn |
| 分子量: | 409.07 |
| 熔 点: | 121~124℃ |
| 密 度: | 1.55 |
| 蒸汽压: | 30℃ |
| 溶解性: | 几乎不溶于水,微溶于大多数有机溶剂 |
| 稳定性: | 暴露于空气和阳光下较易分解,干燥时稳定,遇水分解在 |
| 外观与性状: | 白色结晶,无味 |
| 危险标记: | 15(有害品,远离食品) |
| 用 途: | 用作农用杀菌剂 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:主要引起神经系统的损害。其临床表现有头痛、头晕、精神萎靡、乏力、恶心、食欲减退等。对皮肤可引起接触性皮炎、过敏性皮炎。长期接触,可引起神经衰弱综合征。
二、毒理学资料及环境行为
毒性:属中等毒类。
急性毒性:LD50125mg/kg(大鼠经口);81.3mg/kg(小鼠经口); 人吸入125mg/kg,2小时,呼吸系统,神经系统和胃肠道功能有变化;人经皮肤接触125mg/kg2天,有皮肤刺激,肝、胆炎症。
亚急性和慢性毒性:豚鼠经口0.25mg/kg×90天,血液系统和中枢神经系统改变,死亡。
危险特性:遇明火、高热可燃。受高热分解放出有毒的气体。
燃烧(分解)产物:一氧化碳、二氧化碳、氧化锡。
3.现场应急监测方法:
4.实验室监测方法:
定量测定三苯基锡杀菌剂的生物色谱法{刊,英]/Adinarayana M.;Singh U.S.;Dwivedi T.S.//J.Chromatogr.-1988,435(1).-210~218 《分析化学文摘》1989.6
5.环境标准:
美国 车间卫生标准 0.1mg/m3(皮)(锡)
食物中最高残留限值(特定农作物):0.1-1.0mg/kg(1978)
6.应急处理处置方法:
一、泄漏应急处理
隔离泄漏污染区,周围设警告标志,建议应急处理人员戴自给式呼吸器,穿化学防护服。避免扬尘,小心扫起,收集运至废物处理场所。也可以用大量水冲洗,经稀释的洗水放入废水系统。对污染地带进行通风。如大量泄漏,收集回收或无害处理后废弃。
二、防护措施
呼吸系统防护:可能接触毒物时,应该佩戴防毒口罩。空气中浓度较高时,佩戴自给式呼吸器。
眼睛防护:可采用安全面罩。
身体防护:穿相应的防护服。
手防护:戴防护手套。
其它:工作现场严禁吸烟、进食和饮水。工作后,淋浴更衣。工作服不要带到非作业场所,单独存放被毒物污染的衣服,洗后再用。注意个人清洁卫生。
三、急救措施
皮肤接触:用肥皂水及清水彻底冲洗。就医。
眼睛接触:拉开眼睑,用流动清水冲洗15分钟。就医。
吸入:脱离现场至空气新鲜处。就医。
食入:误服者,饮适量温水,催吐。就医。
灭火方法:泡沫、干粉、砂土。
制备方法与用途
毒性
大鼠急性经口LD50为125毫克/千克,大鼠急性经皮LD50为500毫克/千克。对黏膜有刺激性。野鸡LC50约580毫克/升(8天),蜜蜂则安全。
化学性质纯品为白色晶体,无味,熔点为118~122℃,蒸气压为0.177×10-3帕(30℃),相对密度为1.55。能微溶于大多数有机溶剂,在水中的溶解度约为28毫克/升。置于干燥处稳定,但在空气和阳光下较易分解,最终形成水溶性锡化合物。工业品纯度在90%~95%之间。
用途作为一种保护性杀菌剂,它无内吸作用,主要用于防治马铃薯晚疫病、甜菜褐斑病、大豆炭疽病、褐纹病、紫斑病、水稻稻瘟病、条斑病,胡麻叶枯病、洋葱黑斑病、芹菜叶枯病、菜豆炭疽病等。用量为3~6克/100平方米。除杀菌外,也可用于防治水田中的藻类和水蜗牛。用于甜菜、大豆时还有增糖、增产的作用。用药4周后才可食用或喂饲牲畜。
用途它还可防治甜菜褐斑病、马铃薯晚疫病、大豆炭疽病、黑点病、褐纹病、紫斑病,对水稻稻瘟病、稻曲病、条斑病也有较好的防效。此外,对洋葱黑斑病、芹菜叶枯病、菜豆炭疽病及咖啡的生尾孢病也都有很好的防治效果。特别值得注意的是,它对福寿螺和藻类、水蜗牛有特殊防效。
用途用于防治水稻稻瘟病、稻曲病以及甜菜褐斑病等疾病。
生产方法苯基溴化镁与四氯化锡反应制得三苯基氯化锡,随后与氢氧化钠反应生成三苯基羟基锡,最后通过与乙酰氯的反应制得薯瘟锡。
类别农药
毒性分级高毒
急性毒性口服-大鼠 LD50: 125毫克/千克;小鼠 LD50: 81毫克/千克
可燃性危险特性受热分解可产生有毒含锡气体
储运特性库房应通风干燥,并与食品原料分开储存运输
灭火剂砂土、干粉、泡沫
职业标准时间加权平均容许浓度(TWA)0.1毫克/立方米;短时间接触容许浓度(STEL)0.2毫克/立方米
反应信息
-
作为反应物:描述:三苯基乙酸锡 以 N,N-二甲基乙酰胺 为溶剂, 以91%的产率得到六苯基二锡参考文献:名称:Electrosynthetic aspects of organotin compounds摘要:DOI:10.1016/s0022-328x(00)85658-2
-
作为产物:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Sn: Org.Verb.2, 1.1.2.15.2, page 359 - 384摘要:DOI:
-
作为试剂:参考文献:名称:A highly stereoselective synthesis of podophyllotoxin and analogues based on an intramolecular Diels-Alder reaction摘要:DOI:10.1021/jo00251a003
文献信息
-
Symmetrization of Phenylmercuric Hydroxides by the Action of Nickel(II) and Cobalt(II) Acetylacetonates. Isolation and Structural Characterization of an Intermediate in This Reaction作者:Manfred Döring、Gabriela Hahn、Michael Stoll、Alexander C. WolskiDOI:10.1021/om960799g日期:1997.4.1diphenylmercury, HgO, and Ni(acac)2(THF)2. With PhHgOH or PhHgSH the symmetrization reactions also occurred when catalytic amounts of Ni(acac)2 were used. In contrast, triphenyltin derivatives (hydroxide, acetate, oxide) on treatment with M(acac)2 in aqueous THF gave the stable complexes [M(acac)2(Ph3SnOH)]2 (M = Co, Ni). The structure of [Ni(acac)2(Ph3SnOH)]2 (2) was also determined by X-ray crystallography通过在环境温度下将PhHgOH掺入M(acac)2的配位域中,可以获得复合加合物[M(acac)2(PhHgOHgPh)] 2(M = Co,Ni)。[Ni(acac)2(PhHgOHgPh)(Et 2 O)] 2(1c)的X射线晶体结构显示了由乙酰丙酮氧和双(苯基汞)氧化物的桥联氧配位的二聚镍配合物。将化合物1c的THF溶液回流,得到二苯基汞,HgO和Ni(acac)2(THF)2。使用PhHgOH或PhHgSH时,当催化量的Ni(acac)2时也会发生对称化反应被使用。相反,在THF水溶液中用M(acac)2处理的三苯锡衍生物(氢氧化物,乙酸盐,氧化物)得到稳定的络合物[M(acac)2(Ph 3 SnOH)] 2(M = Co,Ni)。[Ni(acac)2(Ph 3 SnOH)] 2(2)的结构也通过X射线晶体学确定。
-
Synthesis, characterisation and reactivity of 2-functionalised vinylstannanes作者:T Lébl、J Holeček、M Dymák、D SteinbornDOI:10.1016/s0022-328x(00)00887-1日期:2001.42-functionalised vinylstannanes (E)/(Z)-Ph3SnCR′CHYRn with acetic and chloroacetic acid in CDCl3 proceeded by protodestannylation yielding Ph3SnOOCCH2X (X=H, Cl) and CHR′CHYRn. The results of kinetics measurements reveal that Lewis-basic substituents YRn facilitate the electrophilic cleavage of SnC bonds, and this effect increases with the basicity of the heteroatom Y, i.e. in the order S类型(的官能乙烯基锡烷ë)/(Ž)-Ph 3 SnCR'CHYR Ñ和(ë)/(Ž)-Ph 3的SnO(YR Ñ)CHR'(YR Ñ = NME 2,OET,SMe的,SEt; R'= Ph,Bu(正丁基),Pe(正戊基),H)是通过非催化或Pd催化的氢化锡烷基化反应制备的。通过制备型HPLC分离特定的立体异构体,并使用1 H,13 C和119 Sn-NMR光谱进行全面表征。2-官能化乙烯基锡的反应(E)/(ž)-博士3 SnCR'CHYR Ñ与在CDCl乙酸和氯乙酸3通过protodestannylation获得pH进行3 SnOOCCH 2 X(X = H,C1)和CHR'CHYR Ñ。动力学测量的结果表明,路易斯碱性取代基YR n促进了SnCthe键的亲电裂解,并且这种影响随着杂原子Y的碱度而增加,即S
-
Preparation and infrared characterisation of some new organotin carboxylate polymers作者:A. Roy、A.K. GhoshDOI:10.1016/s0020-1693(00)93838-6日期:1977.1polymers. In this paper we wish to report the preparation of a few new polymers using a relatively convenient route involving cleavage reactions of tin-carbon bonds in triorganotin carboxylates (R$nOCOR where R = phenyl, Ph; Propyl, Pr; Butyl, Bu; Cyclohexyl and R’ = H, CH2, CH,CH,) with mercury(I1) salts such as mercuric halides and acetate. In a typical reaction run, to a solution of triorganotin carboxylate近年来,对含有羧酸酯基团的单有机锡聚合物[1-3]的合成和结构有相当大的兴趣。迄今为止,已经在严格的条件下用羧酸裂解锡-碳键用于合成这些聚合物。在本文中,我们希望报告使用相对方便的途径制备一些新的聚合物,该途径涉及三有机锡羧酸盐中的锡碳键裂解反应(R $ nOCOR,其中R =苯基,Ph;丙基,Pr;丁基,Bu;环己基R'= H,CH2,CH,CH,)和汞(I1)盐,例如卤化汞和乙酸盐。在典型的反应过程中,在搅拌下向羧酸三有机锡在乙醚或苯中的溶液中滴加汞盐(1:1)在乙醚中的溶液。尽管立即形成白色沉淀,将混合物在室温下再搅拌4小时以确保完全反应。除去挥发物,并用热苯萃取残留的固体,这留下了白色的不溶聚合物材料(除了甲酸酯外,直到360'C都是不溶的;含-0COH基团的聚合物在约270“ C时分解)。分馏结晶后的苯萃取物得到三有机锡卤化物和有机汞卤化物。相反,乙酸汞与乙酸三苯锡的反应得到二苯
-
Poly Organotin Acetates against DNA with Possible Implementation on Human Breast Cancer作者:George Latsis、Christina Banti、Nikolaos Kourkoumelis、Constantina Papatriantafyllopoulou、Nikos Panagiotou、Anastasios Tasiopoulos、Alexios Douvalis、Angelos Kalampounias、Thomas Bakas、Sotiris HadjikakouDOI:10.3390/ijms19072055日期:——(1) and R = Ph– (2),were evaluated for their in vitro biological properties. The compounds were characterized via their physical properties and FT-IR, 119Sn Mössbauer, and 1H NMR spectroscopic data. The molecular structures were confirmed by single-crystal X-Ray diffraction crystallography. The geometry around the tin(IV) ion is trigonal bi-pyramidal. Variations in O–Sn–O···Sn′ torsion angles lead对两种已知的式[R3Sn(CH3COO)] n的锡基聚合物,其中R = n-Bu–(1)和R = Ph–(2),进行了体外生物学性质的评估。这些化合物通过其物理性质和FT-IR,119SnMössbauer和1H NMR光谱数据进行表征。分子结构通过单晶X射线衍射晶体学确认。锡(IV)离子周围的几何形状为三角双锥体。O–Sn–O···Sn'扭转角的变化分别导致1和2的曲折和螺旋超分子组装。研究了针对人乳腺腺癌细胞系的体外细胞生存力:与雌激素受体(ERs)呈MCF-7阳性,而与ER和1和2孵育时,对ERs呈阴性的MDA-MB-231。已经研究了它们对正常人胎儿肺成纤维细胞(MRC-5)的毒性。与顺铂相比,化合物1和2对MDA-MB-231的抗增殖活性分别高134倍和223倍。还使用流式细胞术测定了由1或2引起的细胞死亡的类型。从1和2含量增加的溶液中发生的粘度差异,怀疑1和2对CT-DNA
-
The reactions of diborane with aryl-organotin compounds作者:G.M. Pickles、T. Spencer、F.G. Thorpe、A.B. Chopa、J.C. PodestaDOI:10.1016/s0022-328x(00)98667-4日期:1984.1A number of tetraaryltin compounds, Ar4Sn (where Ar = phenyl, o- and p-tolyl, and p-chlorophenyl) and triphenyltin compounds, Ph3SnX (where X = Cl, H, OH, OCOCH3, and OCOCF3) have been treated with diborane in tetrahydrofuran. Transmetallation occurs in which one or more aryl groups are transferred to boron. The organoboron intermediates give phenols upon oxidation and boronic and borinic acids upon
表征谱图
-
氢谱1HNMR
-
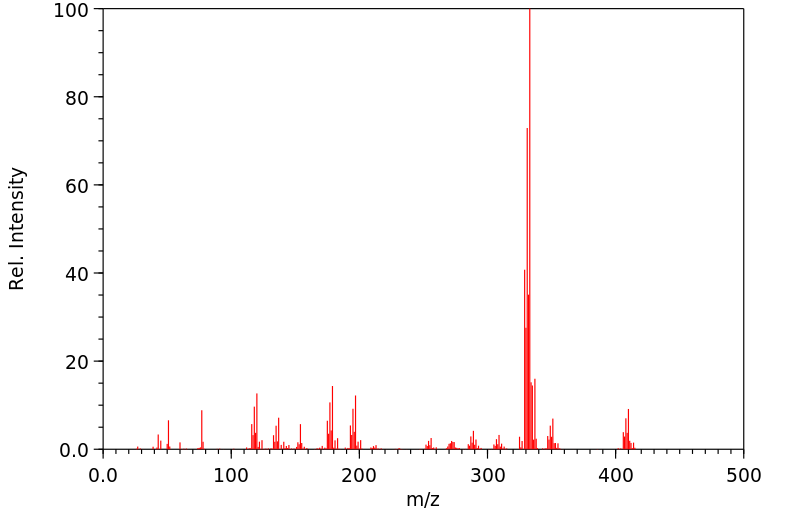
质谱MS
-
碳谱13CNMR
-
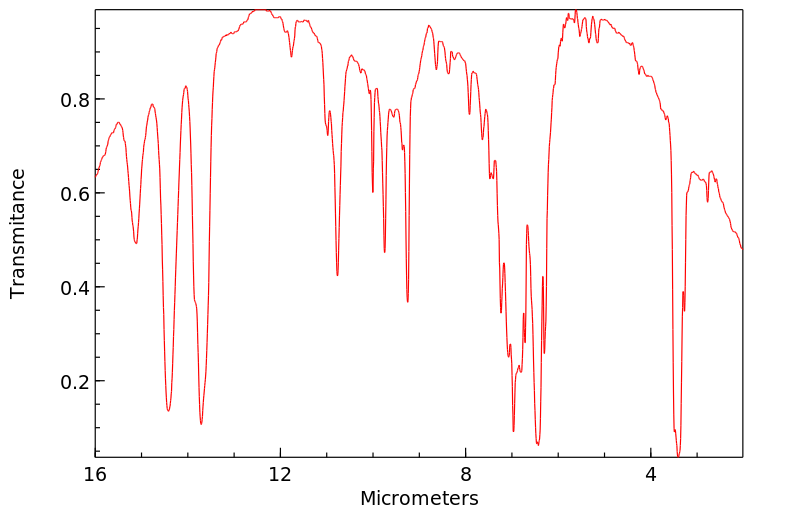
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫