乙基三甲基硅烷 | 3439-38-1
中文名称
乙基三甲基硅烷
中文别名
——
英文名称
trimethylsilylethane
英文别名
ethyltrimethylsilane;2-(trimethylsilyl)ethyl ester;ethyl(trimethyl)silane
CAS
3439-38-1
化学式
C5H14Si
mdl
MFCD01074461
分子量
102.252
InChiKey
UKAJDOBPPOAZSS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:63 °C
-
密度:0.684
-
闪点:<20°C
-
保留指数:571;573;555.3
计算性质
-
辛醇/水分配系数(LogP):2.51
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险类别码:R11
-
危险品运输编号:UN 1993
-
海关编码:2931900090
-
安全说明:S16,S29,S33
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Dolgow; Wolnow, Zhurnal Obshchei Khimii, 1931, vol. 1, p. 91,101-103摘要:DOI:
-
作为产物:描述:(2R,3S,5R)-5-[2-氟-6-(2-三甲基硅烷基乙氧基)嘌呤-9-基]-2-(羟基甲基)四氢呋喃-3-醇 在 溶剂黄146 作用下, 以 二甲基亚砜 、 三乙胺 为溶剂, 反应 7.0h, 生成 乙基三甲基硅烷参考文献:名称:Metabolic Profiling of Chronic Cadmium Exposure in the Rat摘要:A confounding problem with studying the effects of environmental exposure to contaminants in wild populations is that analytical techniques are invasive, particularly where the physiological effects of the toxin are assessed. In this study, a metabonomic approach to investigate the biochemical effects of chronic oral exposure to environmentally realistic doses of CdCl2 (low, 8 mg/kg; high, 40 mg/kg) is presented. H-1 NMR spectra of urine from exposed animals were analyzed using pattern recognition methods to identify biomarkers for a 94 day exposure period. Creatinuria and both increased excretion and complexation of citrate was detected after 19 days of exposure in both exposure groups. This was accompanied by a decrease in plasma Ca2+/Mg2+ ratio in blood plasma after 94 days. Post mortem, magic angle spinning (MAS) H-1 NMR spectroscopy was used alongside conventional analytical techniques to investigate intact tissue directly. According to atomic absorption sectroscopy, kidney tissue accumulated 26.8 +/- 2.5 mug of Cd2+/g dry wt (low) and 75.9 +/- 4.3 mug of Cd2+/g dry wt (high). Using high-resolution MAS H-1 NMR spectroscopy altered lipid content was detected in kidneys from animals exposed to Cd2+. However, unlike acute exposure, no testicular damage was evident. This systemic approach to metabolism demonstrated the different physiological effects of chronic subacute compared with an acute exposure to Cd2+.DOI:10.1021/tx015521u
文献信息
-
Mononuclear calcium complex as effective catalyst for alkenes hydrogenation作者:Xianghui Shi、Cuiping Hou、Lanxiao Zhao、Peng Deng、Jianhua ChengDOI:10.1039/d0cc01745k日期:——Hydrogenolysis of the scorpionate-supported calcium benzyl complex [(TpAd,iPr)Ca(p-CH2C6H4-Me)(THP)] (TpAd,iPr = hydrotris(3-adamantyl-5-isopropyl-pyrazolyl)borate, THP = tetrahydropyran) (2-THP) afforded the mononuclear calcium hydrido complex [(TpAd,iPr)Ca(H)(THP)] (3). Under mild conditions (40 °C, 10 atm H2, 5 mol% cat.), complex 3 effectively catalyzed the hydrogenation of a variety of alkenes
-
Highly Active Superbulky Alkaline Earth Metal Amide Catalysts for Hydrogenation of Challenging Alkenes and Aromatic Rings作者:Johannes Martin、Christian Knüpfer、Jonathan Eyselein、Christian Färber、Samuel Grams、Jens Langer、Katharina Thum、Michael Wiesinger、Sjoerd HarderDOI:10.1002/anie.202001160日期:2020.6.2series of bulky alkaline earth (Ae) metal amide complexes have been prepared: Ae[N(TRIP)2 ]2 (1-Ae) and Ae[N(TRIP)(DIPP)]2 (2-Ae) (Ae=Mg, Ca, Sr, Ba; TRIP=SiiPr3 , DIPP=2,6-diisopropylphenyl). While monomeric 1-Ca was already known, the new complexes have been structurally characterized. Monomers 1-Ae are highly linear while the monomers 2-Ae are slightly bent. The bulkier amide complexes 1-Ae are by far制备了两个系列的大体积碱土金属 (Ae) 金属酰胺配合物:Ae[N(TRIP)2 ]2 (1-Ae) 和 Ae[N(TRIP)(DIPP)]2 (2-Ae) (Ae= Mg、Ca、Sr、Ba;TRIP=SiiPr3,DIPP=2,6-二异丙基苯基)。虽然单体 1-Ca 已为人所知,但新复合物的结构已得到表征。单体1-Ae是高度线性的,而单体2-Ae是轻微弯曲的。体积较大的酰胺配合物 1-Ae 是迄今为止烯烃加氢中最活跃的催化剂,其活性从 Mg 增加到 Ba。催化剂 1-Ba 可以还原环己烯或 3-己烯等内烯烃以及 1-Me-环己烯或四苯乙烯等极具挑战性的底物。它还对芳烃氢化还原蒽和萘(即使被烷基取代)以及联苯具有活性。苯可以还原为环己烷,但未达到完全转化。催化氢化的第一步是形成(酰胺)AeH 物质,它可以形成更大的聚集体。增加酰胺配体的体积会降低聚集体尺寸,但尚不清楚真正的催化剂是什么。 DFT
-
Zero valent iron complexes as base partners in frustrated Lewis pair chemistry作者:Hendrik Tinnermann、Craig Fraser、Rowan D. YoungDOI:10.1039/d0dt03551c日期:——The prototypical iron(0) complex [Fe(CO)3(PMe3)2] (1) forms a frustrated Lewis pair (FLP) with B(C6F5)3 (BCF). In this FLP, the iron complex acts as the Lewis base partner, and the borane as the Lewis acid partner. This FLP is able to cleave H–H, H–Cl, H–O and H–S bonds in H2, HCl, H2O and HSPh. The FLP 1/BCF is shown to catalyze the hydrogenation of alkenes under mild conditions, where terminal alkenes
-
[EN] ANTIBACTERIAL AGENTS<br/>[FR] AGENTS ANTIBACTÉRIENS申请人:ACHAOGEN INC公开号:WO2014165075A1公开(公告)日:2014-10-09Antibacterial compounds of formula (I) are provided, as well as stereoisomers and pharmaceutically acceptable salts and esters thereof; pharmaceutical compositions comprising such compounds; methods of treating bacterial infections by the administration of such compounds; and processes for the preparation of such compounds.提供了化学式(I)的抗菌化合物,以及其立体异构体和药学上可接受的盐和酯;包含这种化合物的药物组合物;通过给予这种化合物治疗细菌感染的方法;以及制备这种化合物的方法。
-
ハロシラン化合物を原料とするアリールシラン化合物の製造方法申请人:国立研究開発法人産業技術総合研究所公开号:JP2020007227A公开(公告)日:2020-01-16【課題】製造コストの低いアリールシラン化合物の製造方法の提供。【解決手段】ニッケル触媒、ルイス酸触媒、及び有機塩基の存在下で、一般式(A−1)、(A−2)、又は(A−3)で表されるハロシラン化合物とアリールボロン酸ピナコールエステルとをクロスカップリング反応させる反応工程を含む、アリールシラン化合物の製造方法。(Rは、各々独立して芳香族炭化水素基、芳香族複素環基、又はC1〜20の炭化水素基;Xは、各々独立してハロゲノ基又はトリフルオロメタンスルホニルオキシ基を表す。)【選択図】なし
表征谱图
-
氢谱1HNMR
-
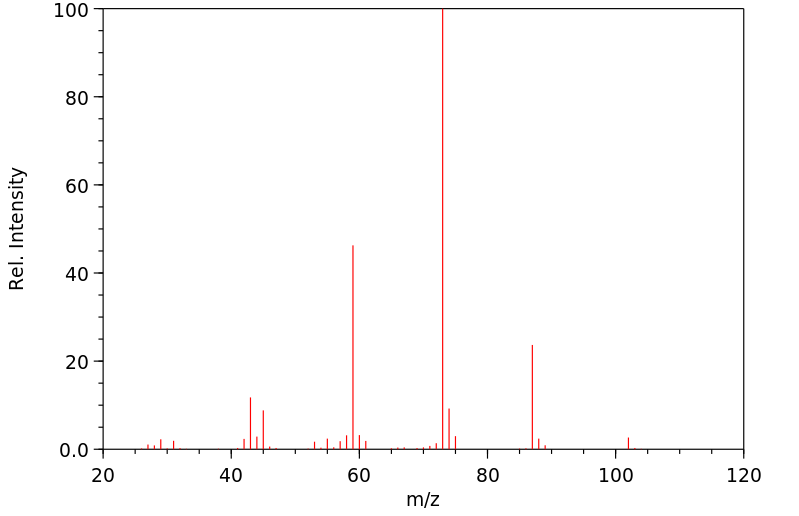
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
镁己烷
锌,二环己基-
锂,3-辛炔基-
锂,(1-苯基乙基)-
铜(I)己基乙炔化物
铜(1+),2-甲基丙烷
铅杂鎓,二乙基甲基-
钠,(1,2,3,4-四甲基-2,4-环戊二烯-1-基)-
钛(4+)四(2,2-二甲基丙烷-1-I去)
邻苯二甲酰基
邻甲基二苯甲酮自由基阳离子
辛烷钠
苄基铜
苄基钠
脱羰秋水仙碱
胂,二(2,2-二甲基丙基)-
纳米碳化钛
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
碳化锆
碳化铪
碳化铌
碳化铀
碳化钽
碳化钒
碘二氟甲基(1+)
硼化二铬
硫代秋水仙碱
硅烷,二甲基丙基-
硅烷,乙基二甲基-2-丙烯基-
硅烷,乙基二(3-甲基丁基)-
石墨溴化物
甲烷,钼
甲基锡烷
甲基铍氢化物
甲基辛基硅烷
甲基硅烷基阳离子
甲基硅烷
甲基二乙烯基硅烷
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基三烯丙基硅烷
甲基三正辛基硅烷
甲基三正己基硅烷
甲基三乙基硅烷
甲基三-N-癸基硅烷
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
甲基-三-n-丁基硅烷
甲基(三丙基)硅烷