二甲基二乙基硅烷 | 756-81-0
中文名称
二甲基二乙基硅烷
中文别名
二乙基二甲基硅烷
英文名称
diethyldimethylsilane
英文别名
Diethyl-dimethylsilan;Dimethyl-diethyl-silan;Diethyl(dimethyl)silane
CAS
756-81-0
化学式
C6H16Si
mdl
MFCD00041343
分子量
116.279
InChiKey
FJWRGPWPIXAPBJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:95 °C
-
密度:0.7214 g/cm3(Temp: 15 °C)
-
保留指数:694;679.7
计算性质
-
辛醇/水分配系数(LogP):2.74
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙基三甲基硅烷 trimethylsilylethane 3439-38-1 C5H14Si 102.252
反应信息
-
作为反应物:描述:二甲基二乙基硅烷 在 I2 作用下, 以 not given 为溶剂, 生成 Dimethylethyl-jodsilan参考文献:名称:Eaborn, C., Journal of the Chemical Society摘要:DOI:
-
作为产物:参考文献:名称:Dolgow; Wolnow, Zhurnal Obshchei Khimii, 1931, vol. 1, p. 91,101-103摘要:DOI:
文献信息
-
Nickel‐Catalyzed Selective Cross‐Coupling of Chlorosilanes with Organoaluminum Reagents作者:Yuki Naganawa、Haiqing Guo、Kei Sakamoto、Yumiko NakajimaDOI:10.1002/cctc.201900047日期:2019.8.21Nickel‐catalyzed cross‐coupling reactions of chlorosilanes with organoaluminum reagents were developed. An electron‐rich Ni(0)/PCy3 complex was found to be an effective catalyst for the desired transformation. The reaction of dichlorosilanes 1 proceeded to give the corresponding monosubstituted products 2. Trichlorosilanes 4 underwent selective double substitution to furnish the corresponding monochlorosilanes
-
The gas-phase acidities of the alkanes作者:Charles H. DePuy、Scott Gronert、Stephan E. Barlow、Veronica M. Bierbaum、Robert DamrauerDOI:10.1021/ja00188a003日期:1989.3acidities of 15 simple alkanes have been determined in a flowing afterglow-selected ion flow tube (FA-SIFT) by a kinetic method in which alkyltrimethylsilanes were allowed to react with hydroxide ions to produce a mixture of trimethylsiloxide ions by loss of alkane and alkyldimethylsiloxide ions by loss of methane. The reaction is proposed to proceed by addition of hydroxide ion to the silane to form在流动余辉选择离子流管 (FA-SIFT) 中,通过动力学方法测定了 15 种简单烷烃的气相酸度,其中烷基三甲基硅烷与氢氧根离子反应生成三甲基硅氧离子混合物。烷烃和烷基二甲基硅氧烷离子通过失去甲烷。该反应建议通过向硅烷中加入氢氧根离子以形成五配位硅酸根离子中间体,该中间体通过两种过渡态分解,一种是在甲基上带负电荷,另一种是在甲基上带负电荷。烷基。产生的硅氧根离子的比例被认为与甲基和烷基阴离子的相对碱度相关。该方法使用已知的甲烷酸度 (/Delta/H/亚酸/) = 416 进行校准。6 kcal/mol 和苯(/Delta/H/度//亚酸/ = 400.7 kcal/mol)。一般而言,发现甲基取代可稳定气相中的烷基阴离子,但发现乙基阴离子比甲基阴离子碱性更强。通过将气相酸度与键解离能结合起来,可以计算出相应烷基的电子亲和力 (EA)。发现许多更简单的烷基自由基具有负的 EA。给出了所研究烷基的结果。«
-
Site‐Isolated Rhodium(II) Metalloradicals Catalyze Olefin Hydrofunctionalization作者:Zihang Qiu、Hao Deng、Constanze N. NeumannDOI:10.1002/anie.202401375日期:2024.4.24Heterogenization of Rh(II) porphyrin catalysts in a metal–organic framework is crucial for catalyst turnover in thermal olefin hydrosilylation and hydrogermylation. Unlike MOF-supported metalloradical catalysts, homogeneous analogues convert to closed shell species that obstruct catalytic turnover. Site-isolated Rh(II) metalloradicals are readily accessed via MOF synthesis with a linker containing
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Si: MVol.C, 68, page 188 - 190作者:DOI:——日期:——
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Si: MVol.C, 64, page 179 - 181作者:DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
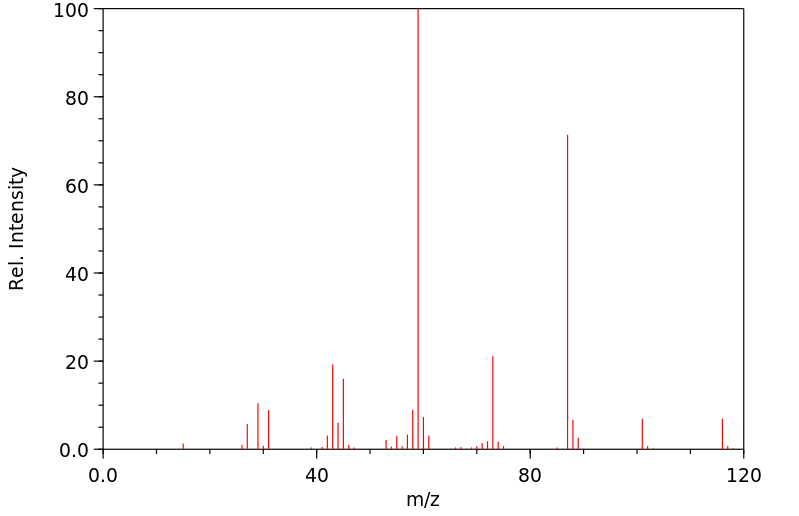
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
镁己烷
锌,二环己基-
锂,3-辛炔基-
锂,(1-苯基乙基)-
铜(I)己基乙炔化物
铜(1+),2-甲基丙烷
铅杂鎓,二乙基甲基-
钠,(1,2,3,4-四甲基-2,4-环戊二烯-1-基)-
钛(4+)四(2,2-二甲基丙烷-1-I去)
邻苯二甲酰基
邻甲基二苯甲酮自由基阳离子
辛烷钠
苄基铜
苄基钠
脱羰秋水仙碱
胂,二(2,2-二甲基丙基)-
纳米碳化钛
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
碳化锆
碳化铪
碳化铌
碳化铀
碳化钽
碳化钒
碘二氟甲基(1+)
硼化二铬
硫代秋水仙碱
硅烷,二甲基丙基-
硅烷,乙基二甲基-2-丙烯基-
硅烷,乙基二(3-甲基丁基)-
石墨溴化物
甲烷,钼
甲基锡烷
甲基铍氢化物
甲基辛基硅烷
甲基硅烷基阳离子
甲基硅烷
甲基二乙烯基硅烷
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基三烯丙基硅烷
甲基三正辛基硅烷
甲基三正己基硅烷
甲基三乙基硅烷
甲基三-N-癸基硅烷
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
甲基-三-n-丁基硅烷
甲基(三丙基)硅烷