偏氟乙烯 | 75-38-7
物质功能分类
中文名称
偏氟乙烯
中文别名
偏二氟乙烯;1,1-二氟乙烯(偏氟乙烯);1,1-二氟乙烯;1,1-二氟乙烯;5,8-二羟基-1,4-萘醌;1,1-二氟乙烯;偏二氟乙烯;制冷剂R-1132a
英文名称
Vinylidene fluoride
英文别名
vinylidene difluoride;1,1-difluoroethylene;VDF;1,1-difluoroethene
CAS
75-38-7
化学式
C2H2F2
mdl
MFCD00000448
分子量
64.0347
InChiKey
BQCIDUSAKPWEOX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−144 °C(lit.)
-
沸点:−83 °C(lit.)
-
密度:0,617 g/cm3
-
蒸气密度:2.2 (vs air)
-
介电常数:3.0(Ambient)
-
物理描述:1,1-Difluoroethylene (or vinylidene fluoride) is a colorless gas which is flammable in the ranges of 5.5 to 21%. It is toxic by inhalation and contact. It is slightly soluble in water and soluble in alcohol and ether. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
-
颜色/状态:Colorless gas
-
气味:Nearly odorless
-
闪点:Flammable gas
-
溶解度:Water solubility = 6.3 cu cm/100 g at 25 °C and 10 kPa
-
蒸汽密度:2.2 (NTP, 1992) (Relative to Air)
-
蒸汽压力:3.0X10+4 mm Hg at 25 °C
-
大气OH速率常数:2.10e-12 cm3/molecule*sec
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强酸。
-
避免接触的条件:受热。
-
聚合危害:可能发生聚合。
-
分解产物:氟化氢。
-
-
自燃温度:640 °C
-
分解:The substance decomposes on heating or on burning producing toxic and corrosive fumes including hydrogen fluoride, fluorine and fluorides.
-
粘度:7.7574 pascal-seconds (liquid) at boiling point
-
燃烧热:-6.92X10+08 J/kmol
-
汽化热:1.7712X10+07 J/kmol at melting point
-
表面张力:0.039942 newtons/meter at melting point
-
电离电位:10.29 eV
-
聚合:The substance may polymerize releasing a large amount of heat, with fire or explosion hazard.
-
折光率:Index of refraction: 1.42
-
保留指数:214
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
In 10.3 L closed containers with oxygen replenishment and carbon dioxide removal, and stocked with 3 rats, the rates of Vinylidene fluoride metab was 1% that of vinyl chloride.
来源:Hazardous Substances Data Bank (HSDB)
代谢
代谢过程非常缓慢,在约260毫克/立方米(100 ppm)的暴露浓度下会达到饱和。最大代谢速率是氯乙烯的1%,低于氟乙烯的20%。据报道,大鼠暴露于1,1-二氟乙烯会导致尿液中氟化物排泄量的一些增加。
Metabolism proceeded very slowly and was saturable at exposure concentrations of about 260 mg/cu m (100 ppm). The maximal metabolic rate was 1% that of vinyl chloride and less than 20% that of vinyl fluoride. Exposure of rats to vinylidene fluoride has been reported to result in some increase in the urinary excretion of fluoride.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Like other halogenated C1 and C2 compounds that are biotransformed to reactive metabolites, vinylidene fluoride causes changes in rat intermediary metabolism which lead to increased exhalation of acetone.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
No epidemiological data relevant to the carcinogenicity of vinylidene fluoride were available. There is inadequate evidence for the carcinogenicity of vinylidene fluoride in experimental animals. Overall evaluation Vinylidene fluoride is not classifiable as to its carcinogenicity to humans (Group 3).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A4:不能归类为人类致癌物。
A4: Not classifiable as a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:偏氟乙烯
IARC Carcinogenic Agent:Vinylidene fluoride
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:无法归类其对人类致癌性
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第39卷:(1986年)一些用于塑料和橡胶的化学品
增补第7卷:致癌性的总体评估:更新国际癌症研究机构专著第1至42卷,1987年;440页;ISBN 92-832-1411-0(已绝版)
第71卷:(1999年)对一些有机化学品、肼和过氧化氢(第一部分、第二部分、第三部分)的再评估
IARC Monographs:Volume 39: (1986) Some Chemicals Used in Plastics and Elastomers
Volume Sup 7: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, 1987; 440 pages; ISBN 92-832-1411-0 (out of print)
Volume 71: (1999) Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide (Part 1, Part 2, Part 3)
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
吸入研究相对较多。代谢消除是一个可饱和的、剂量依赖性的过程。在大气浓度超过饱和点的条件下,消除遵循零级法则,否则遵循正常的一级动力学。
Inhalation comparatively studied. Metabolic elimination was saturable, dose-dependent process. Elimination determined by zero-order law at atmospheric concn above point of saturation, and otherwise by normal first-order kinetics.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
...对250、3750或15000 ppm的vinylidene fluoride暴露6小时后,/B6C3F1/小鼠的血液中vinylidene fluoride的浓度进行了测量。通过模拟大鼠血液中/this cmpd/水平的生理模型被调整用于小鼠,方法是适当地引入小鼠的生理现实参数(肺泡通气量、心输出量、器官血流量和器官体积),并假设针对大鼠确定的化学特异性参数,如组织/血液分配系数,也可以应用于小鼠。随着暴露浓度的增加,小鼠血液中vinylidene fluoride的稳态水平也随之增加。对于15000和3750 ppm的vinylidene fluoride暴露,实验确定的数据落在生理模型预测的95%置信区间内。对于250 ppm的vinylidene fluoride暴露,实验确定血液中/this cmpd/的值低于模型的预测。模型预测表明,对于小鼠,正如在大鼠中观察到的,vinylidene fluoride的水平会迅速上升,在几分钟内达到稳态,并且在暴露结束时,血液水平会迅速下降。在两个最低浓度下,...在停止暴露15分钟或更长时间后采取的血液中检测不到vinylidene fluoride,这表明暴露后水平在或低于检测限,即4 ug/vinylidene fluoride/ml血液。对于15000 ppm的暴露,vinylidene fluoride在暴露后15分钟内可以在血液中检测到。
... Concentrations of vinylidene fluoride were measured in blood of /B6C3F1/ mice during 6 hr exposures to nominal concentrations of 250, 3750 or 15,000 ppm vinylidene fluoride. A physiological model developed to simulate blood levels of /this cmpd/ in rats was adapted for mice by incorporating physiologically realistic parameters for mice where appropriate ( alveolar ventilation, cardiac output, blood flow to organs, and organ volumes) and by assuming that chemical specific parameters such as tissue/blood partition coefficients determined for rats could also be applied to mice. Measured steady state levels of vinylidene fluoride in blood of mice increased with increasing exposure concn. For both the 15,000 and 3750 ppm vinylidene fluoride exposures, the experimentally determined data fell within the 95% confidence interval predicted by the physiological model. For the 250 ppm vinylidene fluoride exposure, the experimentally determined values /for this cmpd/ in blood were lower than what was predicted by the model. Model predictions indicated that for mice, as observed for rats, levels of vinylidene fluoride would rise very rapidly, reaching steady state within minutes of exposure, and that at the end of exposure, blood levels will decline rapidly. At the two lowest concn, ... no vinylidene fluoride /could be detected/ in blood taken 15 min or longer after cessation of exposure, suggesting that the post exposure levels were at or below the limit of detection which was 4 ug/vinylidene fluoride/ml blood. For the 15,000 ppm exposure vinylidene fluoride could be detected in blood up to 15 min post exposure.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
一个生理模型被开发出来,用以描述F344/N雄性大鼠在只有鼻子暴露系统中,暴露于27到16,000 ppm浓度的化合物6小时后,对偏氟乙烯的摄取。血液中偏氟乙烯的稳态浓度随着暴露浓度的增加而线性增加...达到稳态浓度的时间很短,在15分钟内发生。暴露结束后,迅速下降也被观察到。偏氟乙烯在不同脂质和水含量组织中的溶解度非常有限。根据开发的模型预测,尽管偏氟乙烯的总代谢量随着暴露浓度的增加而增加,但这种增加与暴露浓度并不是线性关系。尽管血液中偏氟乙烯的水平随着浓度的增加而线性增加,但在大约2000 ppm时,每6小时暴露代谢的偏氟乙烯量接近最大值。
A physiological model was developed to describe the uptake of vinylidene fluoride in male F344/N rats exposed /to the cmpd/ for 6 hr at a concentrations ranging from 27 to 16,000 ppm in a nose only exposure system. The steady state concn of vinylidene fluoride in blood increased linearly with increases in the exposure concn. ... Rise to steady state concentrations was rapid, occurring within 15 min. Rapid decreases following the end of exposures were also noted. Vinylidene fluoride had very limited solubility in tissues with varying lipid and aqueous content. Predictions based on the model developed indicate that while the total amount of /cmpd/ metabolized increased with increasing exposure concn, this increase was not linearly related to exposure concn. Even though the blood levels of vinylidene fluoride increased linearly with increasing concn, the amount of vinylidene fluoride metabolized per 6 hr exposure approached a maximum at about 2000 ppm.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
研究了氟乙烯和氟氯乙烯在体外引起的脱氟作用和细胞色素P450的丢失。从雄性Sprague-Dawley大鼠中分离出肝微粒体,其中一些用钠苯巴比妥或β-萘黄酮进行了预处理。将这些微粒体与1,1-二氟乙烯、2-氯-1,1-二氟乙烯、三氟乙烯或三氟氯乙烯一起孵化。研究了结构对氟离子释放和细胞色素P450及血红素丢失的影响。来自苯巴比妥处理大鼠的微粒体中发生了接近最大限度的氟离子释放。最大的释放发生在2-氯-1,1-二氟乙烯上,其次是三氟氯乙烯、三氟乙烯和1,1-二氟乙烯。释放的氟离子量是从未处理微粒体中释放的2.4到4倍。β-萘黄酮相对于未处理大鼠的微粒体,对所有化合物的氟离子释放略有抑制作用。三氟乙烯导致微粒体中细胞色素和血红素的最大损失,其次是2-氯-1,1-二氟乙烯、三氟乙烯和1,1-二氟乙烯。结果表明,氟乙烯和氯乙烯是细胞色素P450的底物,能有效灭活细胞色素P450同种物。然而,进行这些反应的能力取决于它们的卤素取代程度和性质。
Defluorination and cytochrome p450 loss induced by fluoroethenes and fluorochloroethenes were studied in vitro. Hepatic microsomes were isolated from male Sprague-Dawley rats, some of which had been pretreated with sodium phenobarbital or beta-naphthoflavone. These were incubated with 1,1-difluoroethene, 2-chloro-1,1-difluoroethene, trifluoroethene or trifluorochloroethene. The effects of structure on fluoride release and cytochrome p450 and heme loss were investigated. Near maximal release of fluoride occurred in microsomes from phenobarbital treated rats. The greatest release occurred with 2-chloro-1,1-difluoroethene, followed by trifluorochloroethene, trifluoroethene and 1,1-difluoroethene in that order. The amounts of fluoride released were 2.4 to 4 times that released from untreated microsomes. beta-Naphthoflavone caused a slight inhibition of fluoride release from all compounds, relative to microsomes from untreated rats. Trifluoroethene caused the greatest loss of cytochrome and heme from microsomes, followed by 2-chloro-1,1-difluoroethene, trifluoroethene, and 1,1-difluoroethene in that order. /Results indicate/ that fluoroethenes and chloroethenes are cytochrome p450 substrates that effectively inactivate cytochrome p450 isozymes. The ability to undergo these reactions, however, depends on the degree and nature of their halogen substituents.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:D
-
职业暴露限值:TWA: 1 ppm, Ceiling: 5 ppm [use 1910.1017]
-
TSCA:T
-
危险等级:2.1
-
危险品标志:F,F+
-
安全说明:S16,S7/9
-
危险类别码:R12
-
WGK Germany:3
-
危险品运输编号:UN 1959 2.1
-
RTECS号:KW0560000
-
包装等级:O52
-
危险类别:2.1
-
储存条件:储存注意事项: - 储存于阴凉、通风的易燃气体专用库房。 - 库温不宜超过30℃。 - 远离火种、热源。 - 应与氧化剂、酸类分开存放,切忌混储。 - 采用防爆型照明、通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备。
SDS
| 国标编号: | 21031 |
| CAS: | 75-38-7 |
| 中文名称: | 1,1-二氟乙烯 |
| 英文名称: | 1,1-difluoroethylene;vinylidene fluoride |
| 别 名: | 偏二氟乙烯;R1132a |
| 分子式: | C 2 H 2 F 2 ;CH 2 CF 2 |
| 分子量: | 64.0 |
| 熔 点: | -144℃ |
| 密 度: | 相对密度(水=1)0.82(0 |
| 蒸汽压: | |
| 溶解性: | 微溶于水,溶于醇、醚等 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色易燃气体,稍具醚的气味 |
| 危险标记: | 4(易燃气体) |
| 用 途: | 用于制造聚偏氟乙烯、氟橡胶和氟塑料,并可作特殊溶剂 |
2.对环境的影响:
一、健康危害
侵入途径:吸入。
健康危害:本品对身体有害,接触后可引起头痛、头晕、恶心等。
二、毒理学资料及环境行为
毒性:属微毒类。
急性毒性:LC50128000ppm,4小时(大鼠吸入)
致突变性:微粒体致突变:鼠伤寒沙门氏菌50ppm,24小时。
危险特性:与空气混合能形成爆炸性混合物。接触热、火星、火焰或氧化剂易燃烧爆炸。若遇高热,可发生聚合反应,放出大量热量而引起容器破裂和爆炸事故。气体比空气重,能在较低处扩散到相当远的地方,遇明火会引着回燃。
燃烧(分解)产物:一氧化碳、二氧化碳、氟化氢。
3.现场应急监测方法:
4.实验室监测方法:
气相色谱法
5.环境标准:
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至上风处,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源。用工业覆盖层或吸附/吸收剂盖住泄漏点附近的下水道等地方,防止气体进入。合理通风,加速扩散。将漏气的容器移至空旷处,注意通风。如无危险,就地燃烧,同时喷雾状水使周围冷却,以防其它可燃物着火。漏气容器要妥善处理,修复、检验后再用。
二、防护措施
呼吸系统防护:空气中浓度超标时,建议佩戴自吸过滤式防毒面具(半面罩)。
眼睛防护:必要时,戴化学安全防护眼镜。
身体防护:穿防静电工作服。
手防护:戴一般作业防护手套。
其它:工作现场严禁吸烟。避免高浓度吸入。
三、急救措施
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
灭火方法:切断气源。若不能立即切断气源,则不允许熄灭正在燃烧的气体。喷水冷却容器,可能的话将容器从火场移至空旷处。灭火剂:雾状水、普通泡沫、干粉、二氧化碳。
制备方法与用途
化学性质
具有轻微醚臭、无色且可燃气体。凝固点为-144℃,沸点为-85.7℃,熔点为-144.1℃。相对密度在-45.3/4℃时为1.001,在液体状态下的相对密度为0.617。可溶于乙醇和乙醚,微溶于水。临界温度为30.1℃,临界压力为4.29MPa,临界密度为0.417g/cm³。在空气中燃烧极限范围为5.5%-21.3%。
用途1,1-二氟乙烯主要用于生产聚偏氟乙烯。通过将1,1-二氟乙烯以水作为介质,并使用过硫酸钾作为引发剂,同时添加缓冲剂,在聚合釜中于50-95℃的温度和约3MPa的压力下进行聚合反应,得到的粉状聚合物经洗涤、干燥后即为成品。这是氟塑料中的机械强度较高的产品,可用一般热塑性塑料加工方法成型。1,1-二氟乙烯具有极好的耐气候性、化学稳定性和耐热性,适用于化工用管道、管件、阀门、泵和贮槽等衬里及防腐隔膜。在化工机械中可用于制造密封件、齿轮、轴承等部件,在电子电气工业中也有多项重要用途,并可用作特种溶剂。
用途 生产方法-
偏二氟乙烷氯化法:在干燥的乙炔与氢氟酸反应生成1,1-二氟乙烷后,通过压缩、分馏和提纯步骤得到精制的1,1-二氟乙烷。然后将其与定量配比的氯气混合,并于650-680℃条件下充分热解生成粗偏氟乙烯,再经过压缩、分馏和提纯步骤以获得精单体。
-
其他方法:近年来随着氯氟烷替代物工业的发展,可以采用一步反应法使用1,1,1-三氯乙烷制得1,1-二氟-1-氯乙烷,随后通过热裂解去除氯化氢得到所需产物。
压缩气体和液化气体
毒性分级低毒
急性毒性吸入:大鼠LC50为128000PPM/4小时
爆炸物危险特性与空气混合易爆
可燃性危险特性易燃;遇热分解生成有毒氟化氢气体
储运特性应存放在通风、低温和干燥的库房中,远离氧化剂存放
灭火剂用水进行灭火
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三氟乙烯 1,1,2-trifluoroethylene 359-11-5 C2HF3 82.0251 四氟乙烯 polytetrafluoroethylene 116-14-3 C2F4 100.016 氟化乙烯 1-fluoroethylene 75-02-5 C2H3F 46.0442 2-氯-1,1-二氟乙烯 1-Chloro-2,2-difluoroethene 359-10-4 C2HClF2 98.4797 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三氟乙烯 1,1,2-trifluoroethylene 359-11-5 C2HF3 82.0251 四氟乙烯 polytetrafluoroethylene 116-14-3 C2F4 100.016 氟化乙烯 1-fluoroethylene 75-02-5 C2H3F 46.0442 2-溴-1,1-二氟乙烯 2-bromo-1,1-difluoroethene 359-08-0 C2HBrF2 142.931 —— 1,1-difluoropropene 430-63-7 C3H4F2 78.0616
反应信息
-
作为反应物:参考文献:名称:Manufacture of dichlorodifluoro-methane摘要:公开号:US02606937A1
-
作为产物:参考文献:名称:Materials and methods for the conversion of hydrofluorocarbons摘要:本文披露了一种回收有价值的氢氟碳化合物并将其转化为环境无害化合物的方法和材料。更具体地,提供了回收氢氟碳化合物(如HFC-227、HFC-236、HFC-245、HFC-125、HFC-134、HFC-143、HFC-152、HFC-32、HFC-23及其各自的异构体)的方法和材料。提供了将这些氢氟碳化合物转化为氟单体前体(如CFC-217、CFC-216、CFC-215、CFC-115、CFC-114、CFC-113、CFC-112、HCFC-22、CFC-12、CFC-13及其各自的异构体)的过程。提供了将这些氟单体前体转化为氟单体(如HFP、PFP、TFP、TFE和VDF)的材料、方法和方案。公开号:US20040127757A1
-
作为试剂:描述:[1,1,1,3,3,4,4,4-Octafluoro-2-(trifluoromethyl)butan-2-yl] hypobromite 在 偏氟乙烯 作用下, 反应 8.0h, 以20%的产率得到2-(2-Bromo-1,1-difluoroethoxy)-1,1,1,3,3,4,4,4-octafluoro-2-(trifluoromethyl)butane参考文献:名称:全氟烷基次溴酸盐:合成及其与某些氟烯烃的反应性摘要:第一全氟烷基次溴酸盐已经制备由溴(I)氟代硫酸盐的用通式R全氟化叔醇盐反应˚F C(CF 3)2 ONA其中R ˚F CF 3或CF 3 CF 2。这些次溴化物的热稳定性较低,但其行为与类似的次氯酸盐相似,在-20°C以上会迅速分解,得到CF 3 C(O)CF 3和CF 3 Br或CF 3 CF 2 Br。全氟烷基次溴化物与氟代烯烃反应生成的新的聚氟醚的特征为19F和1 H NMR光谱,IR光谱和MS。DOI:10.1016/0022-1139(96)03400-8
文献信息
-
Cross-metathesis reaction of functionalized and substituted olefins using group 8 transition metal carbene complexes as metathesis catalysts申请人:CALIFORNIA INSTITUTE OF TECHNOLOGY公开号:US09403854B2公开(公告)日:2016-08-02The invention pertains to the use of Group 8 transition metal carbene complexes as catalysts for olefin cross-metathesis reactions. In particular, ruthenium and osmium alkylidene complexes substituted with an N-heterocyclic carbene ligand are used to catalyze cross-metathesis reactions to provide a variety of substituted and functionalized olefins, including phosphonate-substituted olefins, directly halogenated olefins, 1,1,2-trisubstituted olefins, and quaternary allylic olefins. The invention further provides a method for creating functional diversity using the aforementioned complexes to catalyze cross-metathesis reactions of a first olefinic reactant, which may or may not be substituted with a functional group, with each of a plurality of different olefinic reactants, which may or may not be substituted with functional groups, to give a plurality of structurally distinct olefinic products. The methodology of the invention is also useful in facilitating the stereoselective synthesis of 1,2-disubstituted olefins in the cis configuration.
-
Titanium-Catalyzed Vinylic and Allylic CF Bond Activation-Scope, Limitations and Mechanistic Insight作者:Moritz F. Kuehnel、Philipp Holstein、Meike Kliche、Juliane Krüger、Stefan Matthies、Dominik Nitsch、Joseph Schutt、Michael Sparenberg、Dieter LentzDOI:10.1002/chem.201201125日期:2012.8.20partially fluorinated alkenes, such as previously unknown (Z)‐1,2‐(difluorovinyl)ferrocene. Mechanistic studies indicate a titanium(III) hydride as the active species, which forms a titanium(III) fluoride by H/F exchange with the substrate. The HDF step can follow both an insertion/elimination and a σ‐bond metathesis mechanism; the E/Z selectivity is controlled by the substrate. The catalysts’ ineffieciency
-
Fluorinated Vinylsilanes from the Copper-Catalyzed Defluorosilylation of Fluoroalkene Feedstocks作者:Hironobu Sakaguchi、Masato Ohashi、Sensuke OgoshiDOI:10.1002/anie.201710866日期:2018.1.2Herein, a copper‐catalyzed C−F bond defluorosilylation reaction of tetrafluoroethylene and other polyfluoroalkenes is described. Mechanistic studies, based on a series of stoichiometric reactions with copper complexes, revealed that the key steps of this defluorosilylation reaction are 1) the 1,2‐addition of a silylcopper intermediate to the polyfluoroalkene and 2) a subsequent selective β‐fluorine
-
Preparation of Tri- and Difluoromethylsilanes via an Unusual Magnesium Metal-Mediated Reductive Tri- and Difluoromethylation of Chlorosilanes Using Tri- and Difluoromethyl Sulfides, Sulfoxides, and Sulfones作者:G. K. Surya Prakash、Jinbo Hu、George A. OlahDOI:10.1021/jo030110z日期:2003.5.1A new and efficient method for the preparation of tri- and difluoromethylsilanes using magnesium metal-mediated reductive tri- and difluoromethylation of chlorosilanes is reported using tri- and difluoromethyl sulfides, sulfoxides, and sulfones. The byproduct of the process is diphenyl disulfide. Since phenyl trifluoromethyl sulfone, sulfoxide, and sulfide are readily prepared from trifluoromethane
-
PYROLYSIS PROCESS申请人:Noelke Joseph Charles公开号:US20050137430A1公开(公告)日:2005-06-23The present invention relates to the pyrolysis of hydrochlorofluorocarbons to form fluoromonomers such as tetrafluoroethylene, the pyrolysis being carried out in a reaction zone lined with nickel and mechanically supported by a jacket of other corrosion resistant metal, the nickel lining providing an improved yield of valuable reaction products.
表征谱图
-
氢谱1HNMR
-
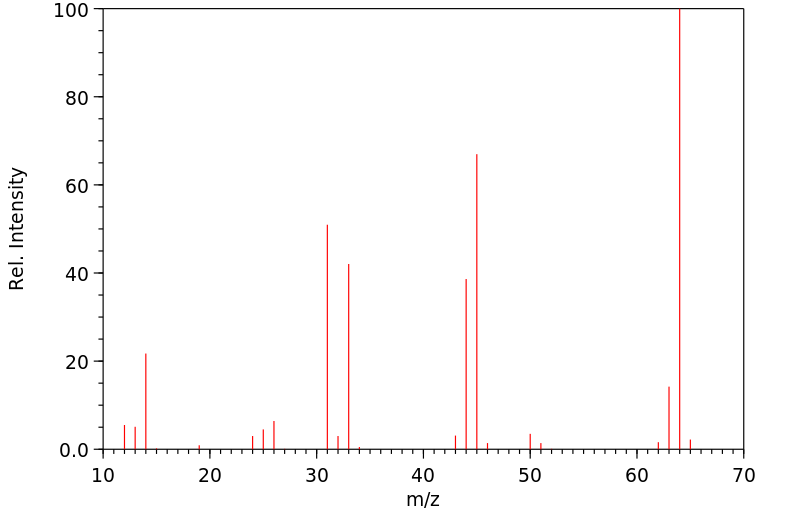
质谱MS
-
碳谱13CNMR
-
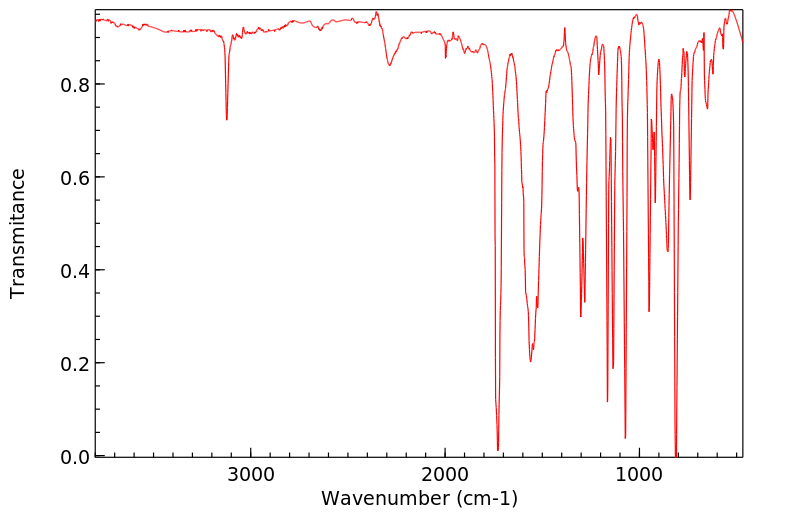
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷