双(三苯基锡)氧化物 | 1262-21-1
中文名称
双(三苯基锡)氧化物
中文别名
六苯基二锡烷;氧化双(三苯基锡)
英文名称
bis(triphenyltin) oxide
英文别名
Bis--oxid;(Ph3Sn)2O;triphenyl(triphenylstannyloxy)stannane
CAS
1262-21-1
化学式
C36H30OSn2
mdl
——
分子量
716.054
InChiKey
MUHFQLVFMFDJOK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:119-123 °C
-
沸点:642.3±38.0 °C(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知的危险反应,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):6.56
-
重原子数:39
-
可旋转键数:8
-
环数:6.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:No
-
危险等级:6.1
-
危险品标志:T,N
-
安全说明:S26,S27,S28A,S45,S60,S61
-
危险类别码:R23/24/25,R50/53
-
海关编码:2934999090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:3146
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | Bis(triphenyltin) oxide Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1262-21-1 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1262-21-1 | Bis(triphenyltin) oxide | 100 | 215-025-4 |
Risk Phrases: 23/24/25 50/53
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed. Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Exposure limits have been recommended for organotin compounds to minimize the potential for adverse effects on immune function and the CNS.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 1262-21-1: United Kingdom, WEL - TWA: (listed as tin organic compounds): 0.1 mg/m3 TWA (except cyhexatin, as Sn) United Kingdom, WEL - STEL: (listed as tin organic compounds): 0.
mg/m3 STEL (except cyhexatin, as Sn) United States OSHA: 0.1 mg/m3 TWA (as Sn) (listed under Tin orga compounds).
Belgium - TWA: (listed as tin organic compounds): 0.1 mg/m3 VLE ( Sn) Belgium - STEL: (listed as tin organic compounds): 0.2 mg/m3 VLE Sn) France - VME: (listed as tin organic compounds): 0.1 mg/m3 VME (a Sn) France - VLE: (listed as tin organic compounds): 0.2 mg/m3 VLE (a Sn) Germany: (listed as tin organic compounds): 0.1 mg/m3 VME (as Sn) Germany: (listed as tin organic compounds): Skin absorber Malaysia: (listed as tin organic compounds): 0.1 mg/m3 TWA (as Sn Netherlands: (listed as tin organic compounds): 0.2 mg/m3 STEL (a Sn) Netherlands: (listed as tin organic compounds): 0.1 mg/m3 MAC (as Spain: (listed as tin organic compounds): 0.1 mg/m3 VLA-ED (as Sn Spain: (listed as tin organic compounds): 0.2 mg/m3 VLA-EC (as Sn Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white to off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 119 - 123 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C36H30OSn2
Molecular Weight: 716.02
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Dust generation, excess heat, confined spaces.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, tin/tin oxides.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1262-21-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Bis(triphenyltin) oxide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ORGANOTIN COMPOUND, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 3146
Packing Group: III
IMO
Shipping Name: ORGANOTIN COMPOUND, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 3146
Packing Group: III
RID/ADR
Shipping Name: ORGANOTIN COMPOUND, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 3146
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T N
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
R 50/53 Very toxic to aquatic organisms, may cause
long-term adverse effects in the aquatic environment.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 27 Take off immediately all contaminated clothing.
S 28A After contact with skin, wash immediately with
plenty of water.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
S 60 This material and its container must be
disposed of as hazardous waste.
S 61 Avoid release to the environment. Refer to
special instructions/safety data sheets.
WGK (Water Danger/Protection)
CAS# 1262-21-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1262-21-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1262-21-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:双(三苯基锡)氧化物 在 HgX2 作用下, 生成 四苯基锡参考文献:名称:金属卤化物对四,五和六配位有机锡化合物的作用:I.卤化汞与双(三苯基锡)氧化物的反应以及一些相关的反应摘要:卤化汞已显示在室温下很容易与双(三苯基锡)氧化物接触,生成苯基汞卤化物,三苯基卤化锡和聚合的二苯基氧化锡,以及少量的四苯基锡。还研究了氯化汞对氢氧化三苯锡钠盐的作用,并为含SnOHg-体系的化合物的不稳定性提供了证据。已证明氧化汞与三苯基氯化锡在沸腾的苯中反应,生成苯基氯化汞和聚合的二苯基氧化锡。对于观察到的反应,提出了可能的机制。DOI:10.1016/s0022-328x(00)86133-1
-
作为产物:描述:三苯基氢氧化锡 以 neat (no solvent) 为溶剂, 生成 双(三苯基锡)氧化物参考文献:名称:锡-I无机和有机金属化合物的热分解。氢氧化三苯锡摘要:摘要研究了三苯基锡氢氧化物在25–400°C温度范围内的热分解。在每个阶段形成的分解产物已被分离和表征。提出了一种涉及还原消除反应的分解方案。该反应和伴随的反应的比例取决于加热方式。DOI:10.1016/s0277-5387(00)84121-2
-
作为试剂:参考文献:名称:An effective method for the preparation of O6 -substituted guanosine and n3-substituted uridine derivatives via the corresponding stannylated intermediates摘要:DOI:10.1016/s0040-4039(00)84907-2
文献信息
-
Polymeric, Molecular and Ionic Organotin Complexes Containing Hypoxanthine, Adenine and 2-Aminopurine. Synthesis and Supramolecular Structures作者:Subrata Kundu、Balaram Mohapatra、Chandrajeet Mohapatra、Sandeep Verma、Vadapalli ChandrasekharDOI:10.1021/cg501322w日期:2015.1.7afforded insoluble intractable products, which, upon addition of dilute HCl in methanol, afforded [Ph2SnCl3(H2O)}(HL3Me)2Cl]·H2O (4) and [(Ph2SnCl4)(HL4Me)2] (5). Complexes 1–5 show an extensive supramolecular organization in the solid state as a result of several intermolecular interactions, prominent among which are the interactions between the nucleobases.L1H [L1H = 3-(N9-次黄嘌呤基)丙酸]与Me 3 SnCl或(n -Bu 3 Sn)2 O反应得到一维配位聚合物[Me 3 Sn(L1)] n(1)和[ n -Bu 3 Sn(L1)] n(2)。L2H [3- N9-(2-氨基嘌呤基)}丙酸]与(Ph 3 Sn)2 O以2:1的比例进行类似反应得到二聚体[(L2)(Ph 3 Sn)L2 Ph 3 Sn(H 2 O)}]·3CH 3 OH·3H 2 O(3)。2-(N9-腺嘌呤基)乙酸(L3H)和3-(N9-腺嘌呤基)丙酸(L4H)与(Ph 3 Sn)2 O的比例为2:1的反应提供了不溶的难处理产品在甲醇中加入稀盐酸,得到[Ph 2 SnCl 3(H 2 O)}(HL3Me)2 Cl]·H 2 O(4)和[(Ph 2 SnCl 4)(HL4Me)2 ](5)。1至5号综合大楼 由于一些分子间的相互作用,显示出广泛的固态超
-
Reaction of organotin oxides, alkoxides and acyloxides with organosilicon hydrides. New preparative method of organotin hydrides作者:Kazuko Hayashi、Jun Iyoda、Isao ShiiharaDOI:10.1016/s0022-328x(00)81719-2日期:1967.10The reaction of organotin oxide, alkoxides and acyloxides with organosilicon hydrides was investigated by varying the mole ratio of the reactants. And it was found that SnO was easily cleaved by SiH giving SnH. Several organotin hydrides, such as tributyltin hydride, tripropyltin hydride and dibutyltin dihydride were obtained in good yields. For triphenyltin hydride and dimethyltin dihydride, it
-
The interaction of organotin(iv) acceptors with 1,4-bis(5-hydroxy-1-phenyl-3-methyl-1H-pyrazol-4-yl)butane-1,4-dioneCoordination chemistry of bis(pyrazolones): a rational design of nuclearity tailored polynuclear complexes. Part 2.22作者:Claudio Pettinari、Fabio Marchetti、Riccardo Pettinari、Augusto Cingolani、Andrei Drozdov、Sergei TroyanovDOI:10.1039/b106665j日期:2002.1.9From the interaction of organotin(IV) halides SnR2Cl2 with 1,4-bis(5-hydroxy-1-phenyl-3-methyl-1H-pyrazol-4-yl)butane-1,4-dione (Q2QH2) in methanol in the presence of base the complexes [SnR2(Q2Q)] (1: R = isobutyl (Bui); 2: R = n-octyl (Ot); 3: R = n-dodecyl (Do)) have been synthesised. The reaction between equimolar quantities of R2SnO and Q2QH2 in toluene yields the dinuclear derivatives [SnR2(Q2Q)]24从有机锡(IV)卤化物SnR 2 Cl 2与1,4-双(5-羟基-1-苯基-3-甲基-1 H-吡唑-4-基)丁烷-1,4-二酮(Q2QH 2)在碱存在下于甲醇中的络合物[SnR 2(Q2Q)](1:R =异丁基(Bu i); 2:R =正辛基(Ot); 3:R =正十二烷基(Do) )已综合。等摩尔量的R 2 SnO和Q2QH 2在甲苯中的反应生成双核衍生物[SnR 2(Q2Q)] 2 4(R = Me)和5(R = Bu n)在溶液中具有顺式-R 2 Sn构型,而在KOH存在下,由Q2QH 2与SnMe 2 Cl 2在CH 3 OH中的反应,形成不溶的可能为多核的异构体形式4。Q 2 QH 2和(R 3 Sn)2 O之间的反应产生衍生物[(SnR 3)2(Q2Q)](6:R = Bu n;7:R = Ph)。6与水反应生成水络合物[(SnBu n 3)2(Q2Q)(H2 O)] 8。已经确定了[SnBu
-
Reactions of [η5-carboxycyclopentadiene][η4-tetraphenylcyclo butadiene] cobalt with alkyl and aryl tin oxides: Synthesis, structural studies and electrochemistry of novel monomeric and dimeric [η5-carboxycyclopentadiene][η4-tetraphenylcyclobutadiene]cobalt based stannoxanes作者:Muthiah Senthil Kumar、Shailesh Upreti、Hanuman P. Gupta、Anil J. EliasDOI:10.1016/j.jorganchem.2006.07.015日期:2006.11out and compared with similar ferrocene carboxylic acid derivatives. The structures and electrochemistry of these compounds are compared with analogous organotin ferrocene carboxylates. The results obtained from the reaction of 1 with alkyl and aryl tin oxides suggest that the formation of stannoxanes assemblies having more than two carboxylate units are not favored indicating that 1 is a highly sterically的反应[η 5 -carboxycyclopentadienyl] [η 4 -tetraphenylcyclobutadiene]钴中,Ph 4 Ç 4的CoC 5 ħ 4 COOH(1)中,用(PH 3 Sn)的2 O,[(Ñ -Bu)2的SnO] Ñ和( Ph 2 SnO)n在回流的甲苯中导致单体化合物Ph 3 SnOC(O)C 5 H 4 CoC 4 Ph 4(2)和二聚化合物n -Bu 2的形成Sn [OC(O)C 5 H 4 CoC 4 Ph 4 ] 2(3)和Ph 2 Sn [OC(O)C 5 H 4 CoC 4 Ph 4 ] 2(4)。通过机械研磨以固态进行的反应也得到相同的结果。晶体结构测定和化合物的循环伏安研究1,2,3和4已经进行了研究并与类似的二茂铁羧酸衍生物进行了比较。将这些化合物的结构和电化学与类似的有机锡二茂铁羧酸盐进行了比较。由1与烷基和芳基锡氧化物的反应获
-
Multi-Pyrene Assemblies Supported on Stannoxane Frameworks: Synthesis, Structure and Photophysical Studies作者:Subrata Kundu、Ramesh K. Metre、Rajeev Yadav、Pratik Sen、Vadapalli ChandrasekharDOI:10.1002/asia.201400054日期:2014.524‐membered macrocycles. Macrocycle 1 possesses intramolecular π–π stacking interactions. An unusual co‐crystal of two tetrameric ladders in 2 was observed in which one of the components of the co‐crystal is neutral whereas the other is dicationic and two pyrenesulfonate counterions are present to balance the overall charge. In the solid state these compounds reveal rich supramolecular structures. Photophysical有机锡氧烷已被用作制备多发色团的支架。单步合成程序可以制备化合物,其中生色团单元的数量可以从2到6不等。因此,of磺酸(PySO 3 H)或C 16 H 9 CHNC 6 H 3(COOH)2(LH 2)与各种有机锡前体的反应产生了含pyr的有机锡氧烷,即[Ph 3 SnPySO 3 ] 6(1),[(ME 2的Sn)2(μ 3 -O)(μ-OH)PySO 3 }2 (ME 2的Sn)2(μ 3 -O)(μ-OH)H 2 ö} 2 ⋅ 2 PySO 3 ](2),[吨卜2的Sn(OH)PySO 3 } 2 ](3) ,[(ñ BUSN)12(O)14(OH)6 PySO 3 } 2 ](4),和[(ñ卜2 Sn)的L} 3 ] 2 ⋅ ç 6 ħ 5 CH 3(5)。化合物1 - 5通过使用X射线晶体学进行了表征。化合物1和5是24元大环。大环1具有分子内π–π堆积相互作用。观
表征谱图
-
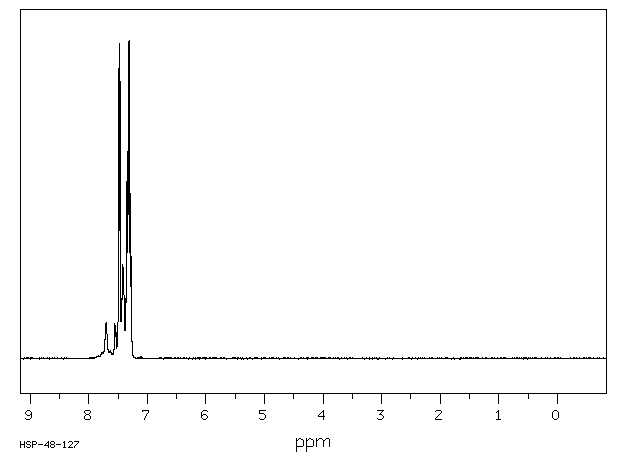
氢谱1HNMR
-
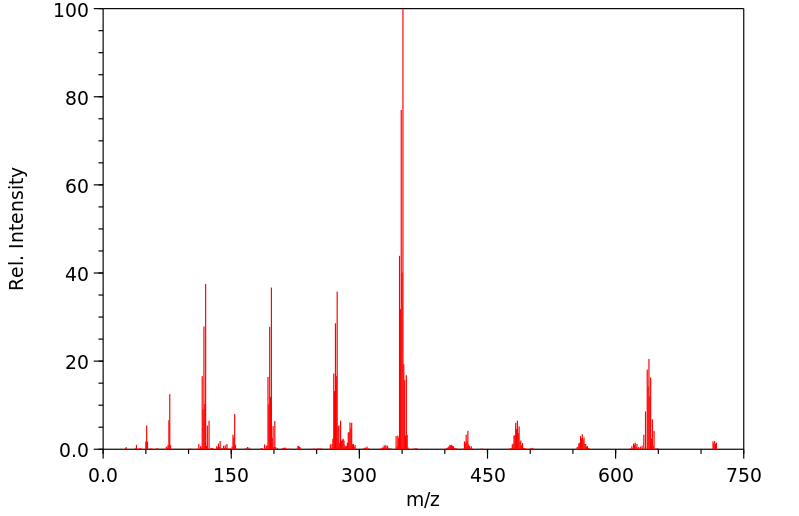
质谱MS
-
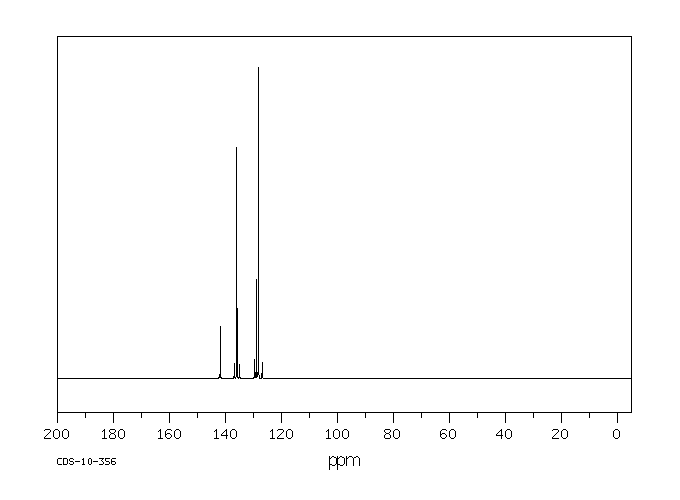
碳谱13CNMR
-
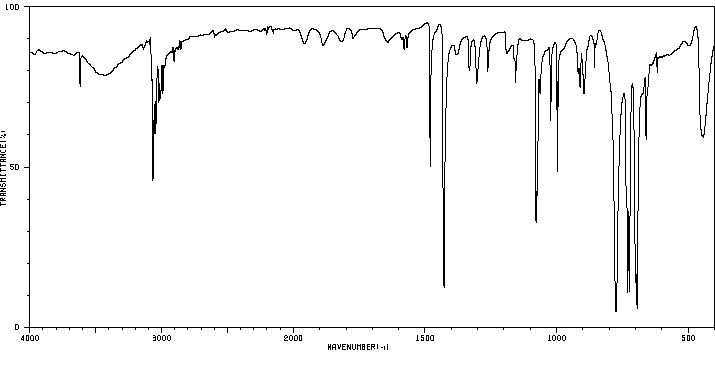
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫