四苯基碘化膦 | 2065-67-0
中文名称
四苯基碘化膦
中文别名
四苯基碘化磷鎓;四苯基膦碘
英文名称
tetraphenylphosphonium iodide
英文别名
tetraphenylphosphanium;iodide
CAS
2065-67-0
化学式
C24H20P*I
mdl
——
分子量
466.301
InChiKey
AEFPPQGZJFTXDR-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.31
-
重原子数:26
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2931900090
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P261,P280,P301+P312
-
危险品运输编号:2811
-
危险性描述:H301,H311,H332
-
储存条件:存储在通风良好、干燥且阴凉的地方,并确保容器密封保存。请将存放地点锁好,远离水源和氧化剂。
SDS
四苯基碘化膦 修改号码:5
模块 1. 化学品
产品名称: Tetraphenylphosphonium Iodide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 四苯基碘化膦
百分比: >97.0%(T)
CAS编码: 2065-67-0
分子式: C24H20IP
四苯基碘化膦 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
光敏, 易湿
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
四苯基碘化膦 修改号码:5
模块 9. 理化特性
外观: 晶体-粉末
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 碘化氢, 磷氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
四苯基碘化膦 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Tetraphenylphosphonium Iodide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 四苯基碘化膦
百分比: >97.0%(T)
CAS编码: 2065-67-0
分子式: C24H20IP
四苯基碘化膦 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
光敏, 易湿
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
四苯基碘化膦 修改号码:5
模块 9. 理化特性
外观: 晶体-粉末
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 碘化氢, 磷氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
四苯基碘化膦 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:参考文献:名称:Wittig; Rieber, Justus Liebigs Annalen der Chemie, 1949, vol. 562, p. 187,190, 192摘要:DOI:
-
作为产物:参考文献:名称:一种基于结晶诱导的有机离子晶体磷光的碘化物特异性检测的简单高效的磷光探针†摘要:由于碘离子的重原子效应引起的普通荧光团的荧光猝灭,因此开发碘离子的发光检测方法非常具有挑战性。在这里,我们揭示了碘化四苯基phosph(TPP I)的独特的结晶诱导的明亮磷光,并开发了一种新型的碘离子时间门控检测方法,以最大程度地减少复杂生物样品的自发荧光。重碘离子的参与和可及的离子相互作用诱导的有机晶体的参与均有助于实现TPP I的非常高的磷光量子产率(0.42)。这种特定的由碘触发的亮有机磷光使得能够以发光开启的方式开发出一种新的定时门控碘离子检测方法,并进一步为基于固体的肉眼检测碘离子提供了一个简便的测试条。固体基质上的三态磷光。该探针在活细胞中碘化物成像中的出色性能以及在双重加密中的有趣用途,说明了高效有机离子晶体在各个领域的广泛应用前景。DOI:10.1039/c8tc04781b
-
作为试剂:描述:环己烯 、 [bis(2-benzoylamino-3-phenylpropoxy)iodo]benzene 在 四苯基碘化膦 作用下, 以 二氯甲烷 、 氯苯 为溶剂, 反应 10.25h, 以70%的产率得到2-benzoylamino-3-phenyl-propionic acid 2-iodo-cyclohexyl ester参考文献:名称:Amino Acid-Derived Iodobenzene Dicarboxylates: Reagents for Oxidative Conversion of Alkenes to Amino Acid Esters摘要:New amino acid-derived iodobenzene dicarboxylates were prepared by the reaction of (diacetoxyiodo)benzene with N-protected natural amino acids. These compounds in the presence of iodide anion can be used as reagents for beta-iodocarboxylation of alkenes leading to the respective amino acid esters.DOI:10.1021/ol0484714
文献信息
-
Catalyst for decarbonylation reaction申请人:UBE INDUSTRIES, LTD.公开号:EP0826658A1公开(公告)日:1998-03-04A catalyst composed of an organic phosphorus compound having a trivalent or pentavalent phosphorus atom and at least one carbon-phosphorus bonding or a combination of the organic phosphorus compound and a halogen atom-containing compound is effective for decarbonylation, that is, for releasing carbon monoxide from a compound containing a moiety of -CO-CO-O- in its molecular structure.
-
Dicarbonyl(η-cyclopentadienyl)iron(<scp>II</scp>) derivatives of pentaborane(9)作者:Norman N. Greenwood、John D. Kennedy、Christopher G. Savory、John Staves、Keith R. TrigwellDOI:10.1039/dt9780000237日期:——2]– which can react with another equivalent of [Fe(η-C5H5)(CO)2I] to give [Fe(η-C5H5)(CO)2}2(2,4-B5H7)](3); this is the first example of a compound with two transition-metal atoms bound to the pentaborane skeleton. Boron-11 and 1H n.m.r. and mass spectroscopic data confirm the structures assigned to (1) and (3); these are consistent with the hypothesis that nido-penta-borate anions act as 2–3-η ligands所述[B 5 ħ 8 ] -与阴离子反应的[Fe(η-C 5 H ^ 5)(CO)2 I],得到的[Fe(2-B 5 ħ 8)(η-C 5 H ^ 5)(CO)2 ](1)。这可以用KH去质子化,得到阴离子的[Fe(2-B 5 ħ 7)(η-C 5 H ^ 5)(CO)2 ] -它可以与其他等效的[Fe(η-C的反应的5 ħ 5)(CO)2 I],得到[的Fe(η-C 5 H ^ 5)(CO)2} 2(2,4-B 5 H 7)](3);这是在戊硼烷骨架上结合有两个过渡金属原子的化合物的第一个例子。硼11和1 H核磁共振和质谱数据证实了分配给(1)和(3)的结构;这与以下假设相符:在16电子过渡金属化合物中,Nido - penta -borate阴离子充当2–3-η配体,在18电子物种中充当2-σ配体。简要讨论了化合物的电子碰撞片段化。
-
METHOD FOR PRODUCING ALPHA-ACYLOXYCARBONYL COMPOUND AND NOVEL ALPHA-ACYLOXYCARBONYL COMPOUND申请人:Ishihara Kazuaki公开号:US20120323014A1公开(公告)日:2012-12-20A method for producing an α-acyloxycarbonyl compound of the present invention includes performing an intermolecular reaction between a carboxylic acid and a carbonyl compound selected from the group consisting of ketones, aldehydes, and esters, which have a hydrogen atom at the α-position, using a hydroperoxide as an oxidizer and an iodide salt as a catalyst precursor, thereby introducing an acyloxy group derived from the carboxylic acid into the α-position of the carbonyl compound.
-
Binuclear Pd(I)–Pd(I) Catalysis Assisted by Iodide Ligands for Selective Hydroformylation of Alkenes and Alkynes作者:Yang Zhang、Sebastian Torker、Michel Sigrist、Nikola Bregović、Paweł DydioDOI:10.1021/jacs.0c09254日期:2020.10.21is driven by a novel activation strategy and features a unique Pd(I)-Pd(I) mechanism, involving an iodide-assisted binuclear step to release the product. This method enables β-selective hydroformylation of a large range of alkenes and alkynes, including sensitive starting materials. Its utility is demonstrated in the synthesis of antiobesity drug Rimonabant and anti-HIV agent PNU-32945. In a broader自 1938 年被发现以来,加氢甲酰化得到了彻底的研究并在工业中得到了广泛的应用(每年超过 107 公吨)。然而,迄今为止,使用成熟的 Rh 或助催化剂精确控制其区域选择性的能力已被证明是难以捉摸的,从而限制了许多具有合成价值的醛的获得。钯催化剂代表了一种有吸引力的替代品,但由于不希望的副加工,它们的使用仍然很少。在这里,我们报告了一种高选择性和异常活性的催化剂系统,该系统由一种新型活化策略驱动,并具有独特的 Pd(I)-Pd(I) 机制,涉及碘化物辅助双核步骤以释放产物。这种方法能够对大范围的烯烃和炔烃(包括敏感的起始材料)进行 β 选择性加氢甲酰化。它的效用在抗肥胖药物利莫那班和抗 HIV 药物 PNU-32945 的合成中得到了证明。在更广泛的背景下,新的机理理解使其他对化学工业具有重要意义的羰基化反应的发展成为可能。
-
[EN] PROCESS FOR PREPARATION OF ALUMINUM SALT OF PHOSPHONIC ACID ESTER<br/>[FR] PROCÉDÉ DE PRÉPARATION D'UN SEL D'ALUMINIUM D'UN ESTER D'ACIDE PHOSPHONIQUE申请人:ICL IP AMERICA INC公开号:WO2013077989A1公开(公告)日:2013-05-30There is provided herein a process for the preparation of aluminum salts of phosphonic acid esters by reacting phosphonic acid diesters with aluminum hydroxide in the presence of a catalyst. The catalyst is selected from the group consisting of a phase transfer catalyst, a thermally stable tertiary amine having a boiling point higher than about 140°C, a thermally stable tertiary phosphine having a boiling point higher than 140°C and combinations thereof. The aluminum phosphonates prepared can be used for making flame retarded thermoplastic polymers.
表征谱图
-
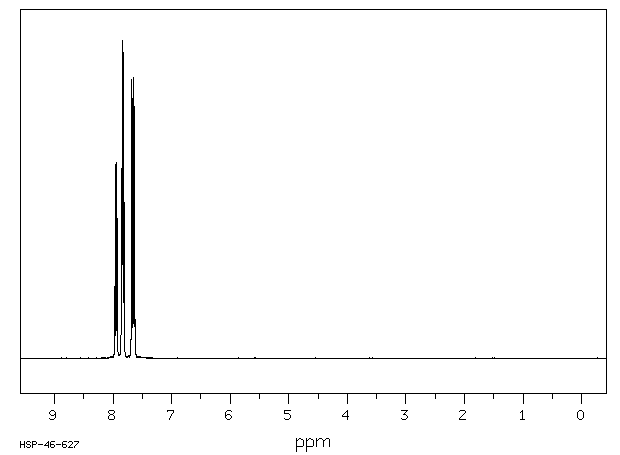
氢谱1HNMR
-
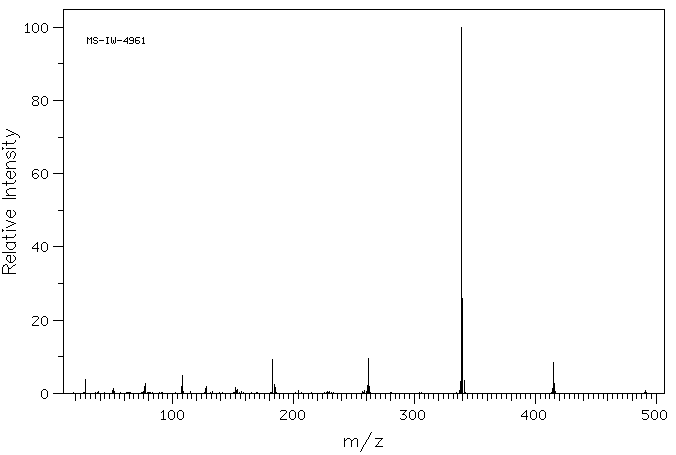
质谱MS
-
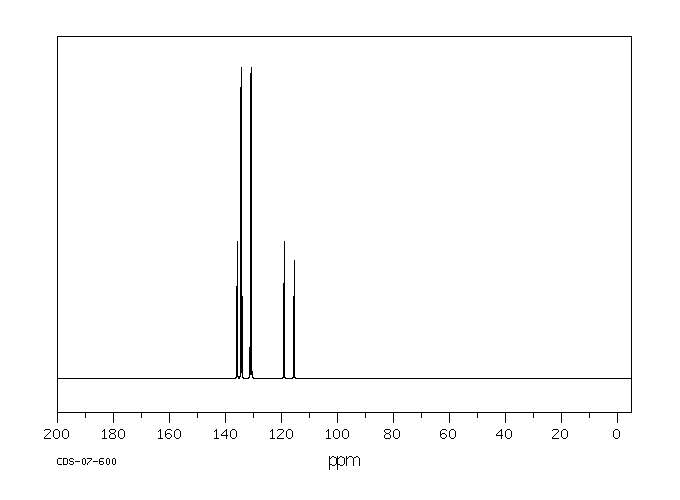
碳谱13CNMR
-
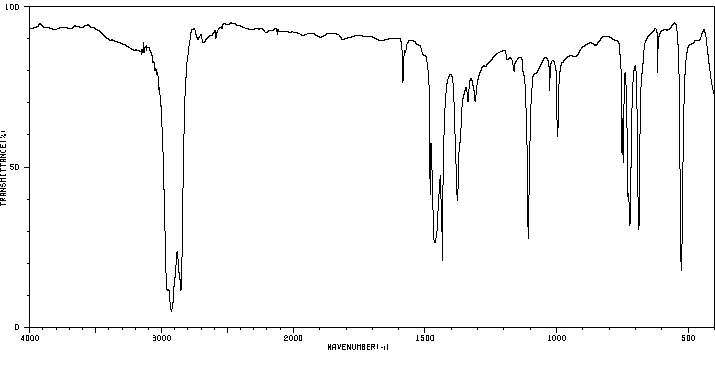
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫