对氨基苯甲醚-3-磺酸 | 13244-33-2
中文名称
对氨基苯甲醚-3-磺酸
中文别名
对茴香胺-2-磺酸;4-甲氧基苯胺-2-磺酸;4-氨基苯甲醚-3-磺酸;2-氨基-5-甲氧基苯磺酸
英文名称
2-amino-5-methoxybenzenesulfonic acid
英文别名
1-amino-4-methoxybenzene-2-sulphonic acid
CAS
13244-33-2
化学式
C7H9NO4S
mdl
MFCD00035804
分子量
203.219
InChiKey
KZKGEEGADAWJFS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:314-318 C
-
密度:1.4540 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:98
-
氢给体数:2
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2922299090
-
危险品运输编号:25kgs
-
RTECS号:DB4950000
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H317,H319
SDS
| Name: | 2-Amino-5-Methoxybenzenesulfonic Acid 98% Material Safety Data Sheet |
| Synonym: | 4-Aminoanisole-3-Sulfonic Acid; 4-Methoxy-2-Sulfoaniline |
| CAS: | 13244-33-2 |
Synonym:4-Aminoanisole-3-Sulfonic Acid; 4-Methoxy-2-Sulfoaniline
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 13244-33-2 | 2-Amino-5-Methoxybenzenesulfonic Acid | 98% | 236-224-2 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May cause methemoglobinemia, cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), convulsions, and death.
Inhalation:
Causes respiratory tract irritation. May cause methemoglobinemia, cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), convulsions, tachycardia, dyspnea (labored breathing), and death. Inhalation may be fatal as a result of spasm, inflammation, edema of the larynx and bronchi, chemical pneumonitis and pulmonary edema. May cause burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting. Can produce delayed pulmonary edema.
Chronic:
May cause methemoglobinemia, which is characterized by chocolate-brown colored blood, headache, weakness, dizziness, breath shortness, cyanosis (bluish skin due to deficient oxygenation of blood), rapid heart rate, unconsciousness and possible death. Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively. For methemoglobinemia, administer oxygen alone or with Methylene Blue depending on the methemoglobin concentration in the blood.
Antidote: Methylene blue, alone or in combination with oxygen is indicated as a treatment in nitrite induced methemoglobinemia.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 13244-33-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H9NO4S
Molecular Weight: 203.1273
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 13244-33-2: DB4950000 LD50/LC50:
CAS# 13244-33-2: Draize test, rabbit, eye: 500 mg/24H Mild; Draize test, rabbit, skin: 500 mg/24H Mild; Oral, rat: LD50 = 10 gm/kg.
Carcinogenicity:
2-Amino-5-Methoxybenzenesulfonic Acid - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 13244-33-2: 1
Canada
CAS# 13244-33-2 is listed on Canada's NDSL List.
CAS# 13244-33-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 13244-33-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-5-羟基萘-1,7-二磺酸 6-amino-1-naphthol-3,5-disulphonic acid 6535-70-2 C10H9NO7S2 319.316 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-amino-5-hydroxy-benzenesulfonic acid 2835-05-4 C6H7NO4S 189.192 —— 2-azido-5-methoxybenzenesulfonic acid 871831-99-1 C7H7N3O4S 229.216 —— 2-[[4-[(4-Amino-2,5-dimethylphenyl)diazenyl]-2,5-dimethylphenyl]diazenyl]-5-methoxybenzenesulfonic acid 888322-60-9 C23H25N5O4S 467.549 7-氨基-4-羟基-3-[(4-甲氧基-2-磺基苯基)偶氮]萘-2-磺酸 Gamma Acid 16452-06-5 C17H15N3O8S2 453.453 —— 2-Azido-5-methoxybenzenesulfonyl chloride 1025996-94-4 C7H6ClN3O3S 247.662
反应信息
-
作为反应物:描述:对氨基苯甲醚-3-磺酸 在 盐酸 、 sodium nitrite 、 sodium iodide 作用下, 以 水 为溶剂, 以40%的产率得到sodium 2-iodo-5-methoxybenzenesulfonate参考文献:名称:Uyanik, Muhammet; Akakura, Matsujiro; Ishihara, Kazuaki, Journal of the American Chemical Society, 2009, vol. 131, p. 251 - 262摘要:DOI:
-
作为产物:描述:苯酚 在 盐酸 、 sodium nitrate 、 硫酸 、 sodium hydroxide 、 锌 作用下, 以 水 为溶剂, 15.0~115.0 ℃ 、1.5 MPa 条件下, 反应 6.09h, 生成 对氨基苯甲醚-3-磺酸参考文献:名称:一种2-氨基-5-甲氧基苯磺酸的合成方法摘要:本发明公开了一种2-氨基-5-甲氧基苯磺酸的合成方法,属于有机化学合成技术领域。本发明首先在苯酚中加入2H2SO4和硝酸钠生成对硝基苯酚,之后再加入锌粉和盐酸,进一步生成对氨基苯酚,接下来将氨基苯酚中滴加浓硫酸和阿拉丁试剂,反应后得2-氨基-5-羟基苯磺酸,随后再通过加入氢氧化钠溶,生成2-氨基-5-钠氧基苯酚酸钠,最后通过在2-氨基-5-钠氧基苯酚酸钠中加入CH3I溶液,从而生成2-氨基-5-甲氧基苯酚酸钠后,高温加入H2SO4溶液,生成本发明的一种2-氨基-5-甲氧基苯磺酸。本实例证明,本发明不仅操作简单易行,没有任何毒性,而且合成的产物容易自行生物降解,且合成收率达到82%以上。公开号:CN105218406A
文献信息
-
[EN] WATER SOLUBLE AND WATER FAST DYES FOR INK JET PRINTING<br/>[FR] COLORANTS HYDROSOLUBLES ET SOLIDES À L'EAU POUR L'IMPRESSION PAR JET D'ENCRE申请人:ILFORD IMAGING CH GMBH公开号:WO2013139485A1公开(公告)日:2013-09-26The invention relates to azo dyes of desulfo-K-acid [CA: 35400-55-6], their salts, a method for the preparation of these dyes, or salts thereof, a liquid phase comprising at least one of these dyes, a method for applying the liquid phase on a substrate, a printed matter and the use of these dyes in water-based inks for inkjet printing, in writing utensil or dyeing solutions for manufacturing color filters for optical and opto-electronic applications.
-
AZO COMPOUND AND DYE POLARIZING FILM CONTAINING THE SAME申请人:Nippon Kayaku Kabushiki Kaisha公开号:EP2045296A1公开(公告)日:2009-04-08Disclosed is an azo compound represented by the formula (1) below, a salt thereof, or a copper complex salt compound thereof. (In the formula, R1 and R2 independently represent a hydrogen atom, a sulfonic acid group, a lower alkyl group or a lower alkoxyl group; R3-R6 independently represent a hydrogen atom, a lower alkyl group or a lower alkoxyl group; R7 represents a lower alkyl group or a lower alkoxyl group; and n represents 0 or 1.)
-
Synthesis, spectroscopic characteristics, dyeing performance and TD-DFT study of quinolone based red emitting acid azo dyes作者:Suvidha Shinde、Nagaiyan SekarDOI:10.1016/j.dyepig.2019.04.028日期:2019.9novel fluorescent heterocyclic acid dyes were prepared by diazotizing various sulphonic acid based amines and coupling with 4-hyrdoxyl-1-methyl-2(1H)-quinolone. Structures of synthesized dyes were well supported by 1H NMR, 13C NMR, Mass spectroscopy, FTIR and elemental analysis. The effect of high molecular weight and heterocyclic moiety were observed to have significant influence on photophysical properties
-
Tetrakisazo Compound, Coloring Matter for Recording, Ink Composition and Colored Article申请人:Ohno Hiroaki公开号:US20070227388A1公开(公告)日:2007-10-04The present invention relates to black coloring matter, and relates to a compound represented by the following formula (1): (wherein A and D independently represent an optionally substituted phenyl group or naphthyl group, respectively, and m and n represent 1 or 2, respectively), and the coloring matter for recording and an ink comprising the same. Said compound exhibits high solubility in a medium containing water as a main component, is stable even when an aqueous dye solution or an ink having a high concentration is prepared therefrom and is stored for a long period of time, has low color rendering property, exhibits colorless and neutral black, provides a printed image having high density and also provides a black recorded image being excellent in moisture fastness, light fastness and an ozone gas fastness, and further can be synthesized easily and can be produced at a low cost.
-
Functionalization of α‐C(sp <sup>3</sup> )−H Bonds in Amides Using Radical Translocating Arylating Groups作者:Niklas Radhoff、Armido StuderDOI:10.1002/anie.202013275日期:2021.2.15α‐C−H arylation of N‐alkylamides using 2‐iodoarylsulfonyl radical translocating arylating (RTA) groups is reported. The method allows the construction of α‐quaternary carbon centers in amides. Various mono‐ and disubstituted RTA‐groups are applied to the arylation of primary, secondary, and tertiary α‐C(sp3)−H‐bonds. These radical transformations proceed in good to excellent yields and the cascades据报道,使用 2-碘芳基磺酰基自由基易位芳基化 (RTA) 基团对 N-烷基酰胺进行 α-C-H 芳基化。该方法允许在酰胺中构建α-季碳中心。各种单取代和二取代的 RTA 基团适用于伯、仲和叔 α-C(sp 3 )-H-键的芳基化。这些自由基转化以良好到优异的产率进行,级联包括 1,6-氢原子转移,随后是 1,4-芳基迁移以及随后的 SO 2挤出。
表征谱图
-
氢谱1HNMR
-
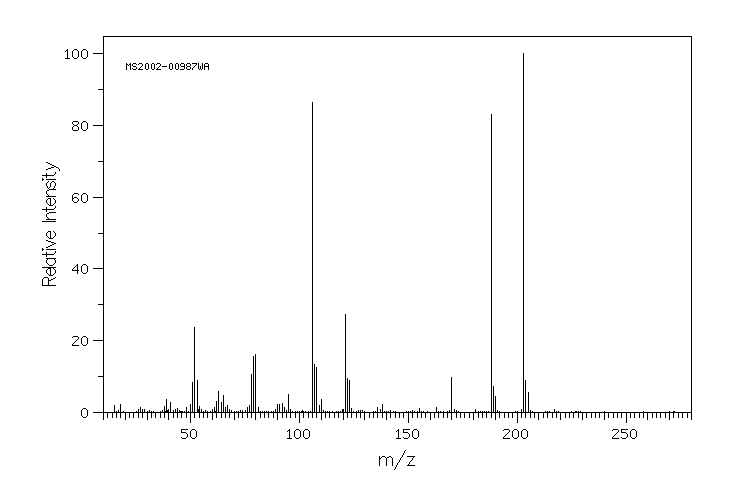
质谱MS
-
碳谱13CNMR
-
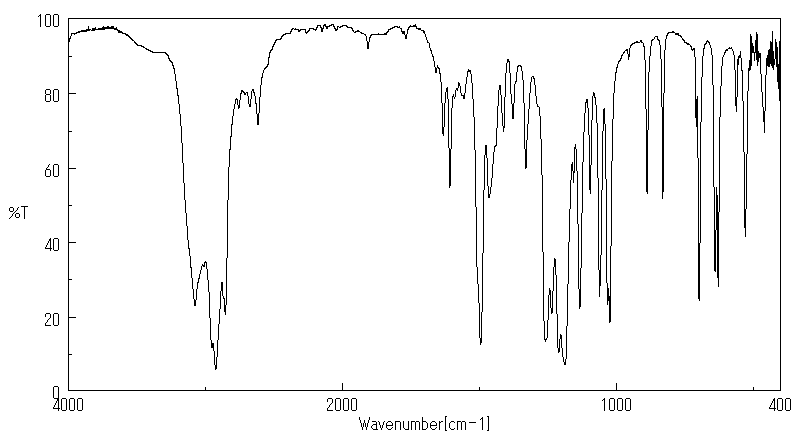
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫