(2S-顺)-四氢化-4-甲基-2-(2-甲基-1-丙烯基)-2H-吡喃 | 3033-23-6
物质功能分类
中文名称
(2S-顺)-四氢化-4-甲基-2-(2-甲基-1-丙烯基)-2H-吡喃
中文别名
左旋玫瑰醚;4-甲基-2-(2-甲基-1-丙烯基)四氢吡喃
英文名称
(2S,4R)-4-methyl-2-(2-methyl-1-propenyl)tetrahydropyran
英文别名
(-)-cis-rose oxide;(2S,4R) rose oxide;cis-(-)-rose oxide;cis-rose oxide;rose oxide;(2S)-4c-Methyl-2r-(2-methyl-propenyl)-tetrahydro-pyran;(2S,4R)-4-methyl-2-(2-methylprop-1-en-1-yl)tetrahydro-2H-pyran;(2S,4R)-4-methyl-2-(2-methylprop-1-enyl)oxane
CAS
3033-23-6
化学式
C10H18O
mdl
——
分子量
154.252
InChiKey
CZCBTSFUTPZVKJ-NXEZZACHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70-71 °C12 mm Hg(lit.)
-
密度:0.871 g/mL at 20 °C(lit.)
-
闪点:68 °C
-
溶解度:Very slightly soluble in water, soluble in alcohol and oils
-
LogP:3.13
-
物理描述:colourless mobile liquid, powerful, distinctive geranium top note
-
折光率:1.453-1.457
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
危险类别码:R36/38
-
海关编码:2932999099
-
安全说明:S26,S61
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (-)-(S)-Nerol oxide 76936-31-7 C10H16O 152.236
反应信息
-
作为反应物:描述:(2S-顺)-四氢化-4-甲基-2-(2-甲基-1-丙烯基)-2H-吡喃 在 臭氧 、 亚磷酸三乙酯 作用下, 以 四氢呋喃 、 正己烷 为溶剂, 反应 1.25h, 生成 (-)-(2S,4R,E)-Sesquirose oxide参考文献:名称:氧化玫瑰合成倍半乳糖摘要:由四种光学活性的玫瑰氧化物1至4制备八种立体异构的倍半玫瑰氧化物9至16。给出了光谱数据,旋光度和9至16的嗅觉特性。DOI:10.1002/hlca.19800630627
-
作为产物:描述:3,6-dihydro-4-methyl-2H-pyran-2-carboxylic acid 在 铂 氯化亚砜 、 硫酸 、 氢气 、 二异丁基氢化铝 作用下, 以 四氢呋喃 为溶剂, 反应 18.0h, 生成 (2S-顺)-四氢化-4-甲基-2-(2-甲基-1-丙烯基)-2H-吡喃参考文献:名称:Structure Elucidation, Enantioselective Analysis, and Biogenesis of Nerol Oxide in Pelargonium Species摘要:A novel enantioselective synthesis of nerol oxide (3,6-dihydro-4-methyl-2-(2-methyl-1-propenyl)-2H-pyran) was used for the determination of the absolute configuration at C-2. The order of elution of the enantiomers on octakis-(2,3-di-O-butyryl-6-O-tert-butyldimethylsilyl)-gamma-cyclodextrin in OV 1701-vi as the chiral stationary phase in enantioselective GC was determined as (2R) before (2S). Enantioselective multidimensional GC/MS (enantio-MDGC/MS) was used for the determination of the enantiomeric ratios of nerol oxide in different geranium oils. As a result, in all investigated oils nerol oxide occurs as a racemate. The biogenesis of nerol oxide in Pelargonium species was investigated by feeding experiments using deuterium-labeled neryl glucoside as the precursor. The Pelargonium plants were able to convert the fed precursor into racemic nerol oxide, which has to be considered as a "natural racemate"DOI:10.1021/jf981245m
文献信息
-
METHOD FOR PRODUCING CIS-ROSE OXIDE申请人:Koenigsmann Lucia公开号:US20100311988A1公开(公告)日:2010-12-09The present invention relates to a process for the preparation of cis-2-(2-methylprop-1-enyl)-4-methyltetrahydropyran comprising the catalytic hydrogenation of 2-(2-methylprop-1-enyl)-4-methylenetetrahydropyran in the presence of hydrogen and a heterogeneous catalyst comprising ruthenium on a support and subsequently bringing the compounds obtained in this way into contact with a strongly acidic cation exchanger.
-
PROCESS FOR THE PREPARATION OF CIS-ROSE OXIDE申请人:BASF SE公开号:US20130109867A1公开(公告)日:2013-05-02The present invention relates to a process for the preparation of a composition enriched in cis-2-(2-methylprop-1-enyl)-4-methyltetrahydropyran, comprising the catalytic hydrogenation of 2-(2-methylprop-1-enyl)-4-methylenetetrahydropyran in the presence of hydrogen and a heterogeneous catalyst comprising ruthenium.
-
[EN] METHOD FOR PRODUCING DEHYDRO ROSE OXIDE<br/>[FR] PROCÉDÉ DE PRODUCTION D'OXYDE DE ROSE DÉSHYDRO申请人:BASF SE公开号:WO2014184311A1公开(公告)日:2014-11-20The present invention relates to a method for producing dehydro rose oxide by reacting isoprenol and prenal in the presence of at least one sulfonic acid of formula R1-SO3H as catalyst, wherein R1 is selected from phenyl which carries 2 or 3 C1-C4-alkyl substituents, phenyl which carries one C8-C20-alkyl substituent and optionally also 1 or 2 C1-C4-alkyl substituents, and naphthyl which optionally carries 1 or 2 C1-C4-alkyl substituents.
-
COMPOUNDS FOR PREVENTING, REDUCING AND/OR ALLEVIATING ITCHY SKIN CONDITION(S)申请人:SCHMAUS Gerhard公开号:US20160008297A1公开(公告)日:2016-01-14The present invention primarily relates to the use of one or more specific compounds and/or one or more respective salt(s) thereof for preventing, reducing or alleviating itchy skin condition(s), and/or as PAR-2 antagonist. Furthermore, the present invention relates to compositions (products or, respectively, formulations), in particular for topical administration, preferably cosmetic or pharmaceutical compositions, in particular for preventing, reducing or alleviating one or more itchy skin conditions and/or for providing a PAR-2 antagonistic effect, comprising or consisting of an effect amount of such compound(s) and/or salt(s) and one or more cosmetically and/or pharmaceutically acceptable carriers.本发明主要涉及使用一种或多种特定化合物和/或其一个或多个相应盐来预防、减少或缓解瘙痒皮肤状况,和/或作为PAR-2拮抗剂。此外,本发明涉及组合物(产品或相应的配方),特别是用于局部给药,优选为化妆品或药用组合物,特别是用于预防、减少或缓解一种或多种瘙痒皮肤状况和/或产生PAR-2拮抗作用的组合物,包括或由效果量的这种化合物和/或盐以及一个或多个化妆品和/或药用可接受载体组成。
-
Synthesis and odor of optically active rose oxide作者:Takeshi Yamamoto、Hiroyuki Matsuda、Yasuhide Utsumi、Toshimitsu Hagiwara、Tsuneyoshi KanisawaDOI:10.1016/s0040-4039(02)02311-0日期:2002.12Stereoselective synthesis of optically active rose oxide (1) by KHSO4 and Pd–BINAP-catalyzed cyclization of (3R)- and (3S)-3,7-dimethyl-6,7-octadien-1-ol (5) is described. One-pot synthesis of the (3R)- and (3S)-allenyl alcohol 5 from (3R)- and (3S)-citronellol (2) (98% ee) is also described. Furthermore, the odor properties of the four diastereomers of 1 are reported.
表征谱图
-
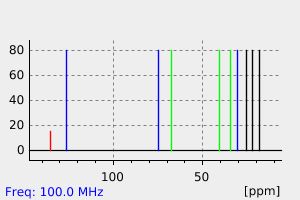
氢谱1HNMR
-
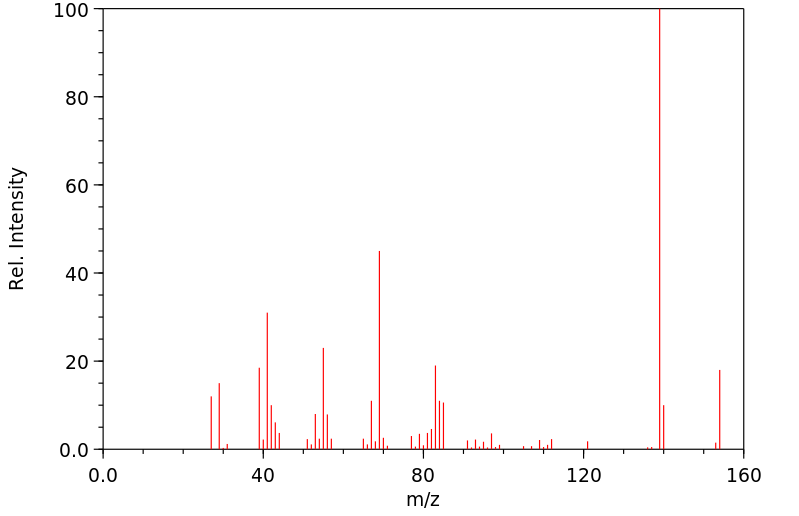
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3S,4R)-3-氟四氢-2H-吡喃-4-胺
鲁比前列素中间体
顺式-3-溴<2-(2)H>四氢吡喃
顺-4-氨基四氢吡喃-3-醇
顺-4-(四氢吡喃-2-氧)-2-丁烯-1-醇
顺-3-Boc-氨基-四氢吡喃-4-羧酸
锡烷,三丁基[3-[(四氢-2H-吡喃-2-基)氧代]-1-炔丙基]-
螺[金刚烷-2,2'-四氢吡喃]-4'-醇
蒿甲醚四氢呋喃乙酸酯
蒜味伞醇B
蒜味伞醇A
茉莉吡喃
苯基2,4-二氯-5-氨磺酰苯磺酸酯
苄基2,3-二-O-乙酰基-4-脱氧-4-C-硝基亚甲基-β-D-阿拉伯吡喃果糖苷
膜质菊内酯
红没药醇氧化物A
红没药醇氧化物
科立内酯
硅烷,(1,1-二甲基乙基)二甲基[[4-[(四氢-2H-吡喃-2-基)氧代]-5-壬炔基]氧代]-
甲磺酸酯-四聚乙二醇-四氢吡喃醚
甲基[(噁烷-3-基)甲基]胺
甲基6-氧杂双环[3.1.0]己烷-2-羧酸酯
甲基4-脱氧吡喃己糖苷
甲基3-脱氧-3-硝基-beta-L-核吡喃糖苷
甲基2,4,6-三脱氧-2,4-二-C-甲基吡喃葡己糖苷
甲基1,2-环戊烯环氧物
甲基-[2-吡咯烷-1-基-1-(四氢-吡喃-4-基)-乙基]-胺
甲基-(四氢吡喃-4-甲基)胺
甲基-(四氢吡喃-2-甲基)胺盐酸盐
甲基-(四氢吡喃-2-甲基)胺
甲基-(四氢-吡喃-3-基-胺
甲基-(四氢-吡喃-3-基)-胺盐酸盐
甲基-(4-吡咯烷-1-甲基四氢吡喃-4-基)-胺
甲基(5R)-3,4-二脱氧-4-氟-5-甲基-alpha-D-赤式-吡喃戊糖苷
环氧乙烷-2-醇乙酸酯
环己酮,6-[(丁基硫代)亚甲基]-2,2-二甲基-3-[(四氢-2H-吡喃-2-基)氧代]-,(3S)-
环丙基-(四氢-吡喃-4-基)-胺
玫瑰醚
独一味素B
溴-六聚乙二醇-四氢吡喃醚
氯菊素
氯丹环氧化物
氨甲酸,[[(四氢-2H-吡喃-2-基)氧代]甲基]-,乙基酯
氨甲酸,[(4-氨基四氢-2H-吡喃-4-基)甲基]-,1,1-二甲基乙基酯(9CI)
氧杂-3-碳酰肼
氧化氯丹
正-(四氢-4-苯基-2h-吡喃-4-基)乙酰胺
次甲霉素 A
桉叶油醇
无