(Z,E,E)-5-羟基-1,7-二(4-羟基苯基)-1,4,6-庚三烯-3-酮 | 52328-96-8
中文名称
(Z,E,E)-5-羟基-1,7-二(4-羟基苯基)-1,4,6-庚三烯-3-酮
中文别名
——
英文名称
bis-demethoxycurcumin
英文别名
bis(4-hydroxycinnamoyl)methane;(1E,4Z,6E)-5-hydroxy-1,7-bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one
CAS
52328-96-8
化学式
C19H16O4
mdl
——
分子量
308.334
InChiKey
YXAKCQIIROBKOP-HSSGTREWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:553.4±50.0 °C(Predicted)
-
密度:1.319±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:23
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:77.8
-
氢给体数:3
-
氢受体数:4
SDS
反应信息
-
作为反应物:描述:(Z,E,E)-5-羟基-1,7-二(4-羟基苯基)-1,4,6-庚三烯-3-酮 在 palladium on activated charcoal 氢气 作用下, 以 甲醇 、 乙酸乙酯 为溶剂, 反应 2.0h, 以65%的产率得到四氢双脱甲氧基二阿魏酰甲烷参考文献:名称:A comparative study on the antioxidant properties of tetrahydrocurcuminoids and curcuminoids摘要:Several curcuminoids and tetrahydrocurcuminoids (THCs), bearing various hydroxyl and/or methoxy groups on their benzene rings, have been synthesized to study their antioxidant and hydrogen donating capacities using the DPPH method at 25 degrees C in methanol. The results show that the tetrahydrocurcuminoids are in general much more efficient than their curcuminoid analogs if they include a phenol group in meta- or para-position of the linking chain and a neighboring phenol or methoxy group. This efficiency gain of THCs by comparison to curcuminoids was attributed to the presence of benzylic hydrogens involved in the oxidation process of these compounds and not to the beta-diketone moiety in the chain. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2007.06.085
-
作为产物:描述:参考文献:名称:A comparative study on the antioxidant properties of tetrahydrocurcuminoids and curcuminoids摘要:Several curcuminoids and tetrahydrocurcuminoids (THCs), bearing various hydroxyl and/or methoxy groups on their benzene rings, have been synthesized to study their antioxidant and hydrogen donating capacities using the DPPH method at 25 degrees C in methanol. The results show that the tetrahydrocurcuminoids are in general much more efficient than their curcuminoid analogs if they include a phenol group in meta- or para-position of the linking chain and a neighboring phenol or methoxy group. This efficiency gain of THCs by comparison to curcuminoids was attributed to the presence of benzylic hydrogens involved in the oxidation process of these compounds and not to the beta-diketone moiety in the chain. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2007.06.085
文献信息
-
Curcuminoids Form Reactive Glucuronides In Vitro作者:Erika Pfeiffer、Simone I. Hoehle、Stephan G. Walch、Alexander Riess、Anikó M. Sólyom、Manfred MetzlerDOI:10.1021/jf0623283日期:2007.1.1the glucuronic acid moiety at the alcoholic hydroxyl group was formed from the same curcuminoids, but not hexahydro-curcuminoids, by human microsomes. Curcuminoids without a phenolic hydroxyl group gave rise to the aliphatic glucuronide only. The phenolic glucuronides of curcuminoids, but not of hexahydro-curcuminoids, were rather lipophilic and, in part, unstable in aqueous solution, their stability由于姜黄素具有抗炎,抗癌和抗阿尔茨海默氏病的活性,因此备受关注,但对其药代动力学和代谢命运知之甚少。因此,目前已经对姜黄素及其主要的I相代谢产物六氢姜黄素以及各种天然和人工类似物的葡糖醛酸苷化进行了体外研究。根据LC-MS / MS分析,由大鼠和人类肝脏微粒体由姜黄素,六氢姜黄素和其他具有酚羟基的类似物产生的主要葡萄糖醛酸苷为酚醛酸葡萄糖苷。然而,由人微粒体由相同的姜黄素而不是六氢姜黄素形成了在醇羟基上带有葡糖醛酸部分的第二葡糖醛酸。没有酚羟基的姜黄素仅产生脂族葡糖醛酸。姜黄素类的酚类葡糖醛酸而不是六氢姜黄素类的酚类葡糖醛酸具有相当的亲脂性,并且在水溶液中部分不稳定,其稳定性在很大程度上取决于芳族取代基的类型。姜黄素及其天然同源物的酚醛葡萄糖醛酸苷,而不是母体化合物,明显抑制了无细胞条件下微管蛋白的组装,暗示了葡糖醛酸苷的化学反应性。姜黄素主要II期代谢产物的这些新颖性质值得进一步研究。它
-
Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing作者:Daisy R. Sherin、Sherin G. Thomas、Kallikat N. RajasekharanDOI:10.1515/hc-2015-0096日期:2015.12.1
Abstract The conversion of arylaldehydes to 1,7-diaryl-5-hydroxyhepta-1,4,6-trien-3-ones (curcuminoids) and the mechanochemical cyclization of these products to 2,2-difluoro-4,6-bis(β-styryl)-1,3,2-dioxaborines using BF3-Et2O are described. Investigation of the cyanide ion sensing ability of the 2,2-difluoro-4,6-bis(β-styryl)-1,3,2-dioxaborines, in relation to the substituent groups on the aryl ring, showed that a hydroxy susbstituent is required, preferably
para to the intervening carbon bridge. -
一种人工合成姜黄素及其衍生物的方法
-
Versatility of the Curcumin Scaffold: Discovery of Potent and Balanced Dual BACE-1 and GSK-3β Inhibitors作者:Rita Maria Concetta Di Martino、Angela De Simone、Vincenza Andrisano、Paola Bisignano、Alessandra Bisi、Silvia Gobbi、Angela Rampa、Romana Fato、Christian Bergamini、Daniel I. Perez、Ana Martinez、Giovanni Bottegoni、Andrea Cavalli、Federica BellutiDOI:10.1021/acs.jmedchem.5b00894日期:2016.1.28multifactorial maladies such as Alzheimer’s disease (AD). The concurrent inhibition of the validated AD targets β-secretase (BACE-1) and glycogen synthase kinase-3β (GSK-3β) by attacking both β-amyloid and tau protein cascades has been identified as a promising AD therapeutic strategy. In our study, curcumin was identified as a lead compound for the simultaneous inhibition of both targets; therefore, synthetic作为解决诸如阿尔茨海默氏病(AD)等复杂和多因素疾病的有用工具,多目标方法已得到越来越多的接受。通过攻击β-淀粉样蛋白和tau蛋白级联反应,同时抑制经过验证的AD靶标β-分泌酶(BACE-1)和糖原合酶激酶-3β(GSK-3β)被认为是一种有前途的AD治疗策略。在我们的研究中,姜黄素被确定为同时抑制两个靶点的先导化合物。因此,合成努力致力于获得一个新的基于姜黄素的类似物的小图书馆,并获得了许多有效和平衡的双靶标抑制剂。特别地,2,6,和7作为具有神经保护潜力和脑通透性的有前途的候选药物出现的。值得注意的是,对于一些新的化合物的对称二酮和β酮-烯醇互变异构形式被有意分离并测试在体外,使我们深入了解用于BACE-1和GSK-3β抑制的关键要求。
-
COMPOUNDS AND MATRICES FOR USE IN BONE GROWTH AND REPAIR申请人:HUMAN BIOMOLECULAR RESEARCH INSTITUTE公开号:US20160038641A1公开(公告)日:2016-02-11Compositions of small molecules, matrices, and isolated cells including methods of preparation, and methods for differentiation, transdifferentiation, and proliferation of animal cells into the osteoblast blast cell lineage were described. Examples of osteogenic materials that were administered to cells or co-cultured with cells are represented by compounds of Formula II, IV, and VI independently or preferably in combination with a matrix to afford bone cells. Small molecule-stimulated cells were also combined with a matrix, placed with a cellular adhesive or material carrier and implanted to a site in an animal for bone repair. Matrix pretreated with compounds of Formula II, IV, and VI were also used to cause cells to migrate to the matrix that is of use for therapeutic purposes.描述了由小分子、基质和孤立细胞组成的组合物,包括制备方法,以及动物细胞分化、转分化和增殖成骨母细胞谱系的方法。给细胞施加的或与细胞共培养的成骨材料的示例由独立或首选与基质组合的Formula II、IV和VI化合物代表。受小分子刺激的细胞还与基质结合,与细胞粘合剂或材料载体一起植入到动物体内的部位进行骨修复。预先用Formula II、IV和VI化合物处理的基质也被用于导致细胞迁移到用于治疗目的的基质上。
表征谱图
-
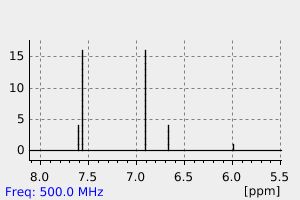
氢谱1HNMR
-
质谱MS
-
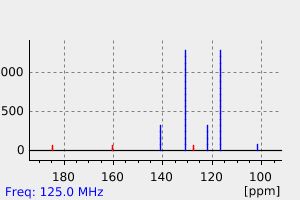
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11aR)-3,7-双(3,5-二甲基苯基)-10,11,12,13-四氢-5-羟基-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂膦酸
龙血素C
顺-1,7-二苯基-1-庚烯基-5-醇
那洛西芬
赤杨酮
赤杨二醇
血竭素
蒙桑酮C
萘-2,7-二磺基酸,钠盐
苯酚,4-(1,3-二苯基丁基)-2-(1-苯基乙基)-
苯甲酸,2-[[2-[(2-羧基苯基)氨基]-5-(三氟甲基)苯基]氨基]-5-[[[(4-羟基-3-甲氧苯基)甲基]氨基]甲基]-
苯基-[4-(2-苯基乙炔基)苯基]甲酮
苯基-[2-[3-(三氟甲基)苯基]苯基]甲酮
苯基-[2-(2-苯基苯基)苯基]甲酮
苯基-(3-苯基萘-2-基)甲酮
苯基-(2-苯基环己基)甲酮
苯,[(二甲基苯基)甲基]甲基[(甲基苯基)甲基]-
苯,1,3-二[1-甲基-1-[4-(4-硝基苯氧基)苯基]乙基]-
脱甲氧姜黄
紫外吸收剂 234
粗糠柴苦素
硫酸姜黄素
矮紫玉盘素
益智醇
白桦林烯酮;1,7-双(4-羟基苯基)-4-庚烯-3-酮
甲酮,苯基(1,6,7,8-四氢-1-甲基-5-苯基环戊二烯并[g]吲哚-3-基)-
甲酮,[3-(4-甲氧苯基)-1-苯基-9H-芴-4-基]苯基-
甲酮,(4-氯苯基)[1-(4-氯苯基)-3-苯基-9H-芴-4-基]-
环香草酮
溴敌隆
波森
桤木酮
桑根酮D
杨梅醇
杨梅酮
杨梅联苯环庚醇-15-葡糖苷
替拉那韦
替吡法尼(S型对映体)
替吡法尼
曲沃昔芬
姜黄素葡糖苷酸
姜黄素beta-D-葡糖苷酸
姜黄素4,4'-二乙酸酯
姜黄素-d6
姜黄素
姜烯酮 A
奈帕芬胺杂质D
四甲基姜黄素
四氢脱甲氧基二阿魏酰甲烷
四氢姜黄素二乙酸酯