1,1,2,2-四氯乙烷 | 79-34-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-43 °C
-
沸点:147 °C(lit.)
-
密度:1.586 g/mL at 25 °C(lit.)
-
蒸气密度:5.8 (vs air)
-
闪点:142-146°C
-
溶解度:水中的溶解度为2830克/升
-
暴露限值:Potential occupational carcinogen. NIOSH REL: TWA 1 ppm (7 mg/m3), IDLH 100 ppm; OSHA PEL: TWA 5 ppm (35 mg/m3); ACGIH TLV: TWA 1 ppm (adopted).
-
介电常数:8.4199999999999999
-
物理描述:1,1,2,2-tetrachloroethane is a colorless to pale yellow liquid with a sweet odor. Sinks in water. (USCG, 1999)
-
颜色/状态:Colorless liquid
-
气味:Sweetish suffocating chloroform-like odor
-
蒸汽密度:5.79 (NTP, 1992) (Relative to Air)
-
蒸汽压力:5.74 mm Hg at 25 °C
-
亨利常数:3.67e-04 atm-m3/mole
-
大气OH速率常数:2.50e-13 cm3/molecule*sec
-
稳定性/保质期:
-
在氯代烃类溶剂中,其溶解能力最强,能与甲醇、乙醇、乙醚、石油醚、苯、四氯化碳、二硫化碳、二甲基甲酰胺等多种有机溶剂完全混溶。它还能溶解油脂、蜡、沥青、煤焦油、樟脑、橡胶、染料、乙基纤维素、硝酸纤维素、聚氯乙烯等多种有机物及硫、磷、卤素、亚硫酸钠等无机物。特别地,120℃时,1,1,2,2-四氯乙烷能溶解100g硫;在25℃时,它在水中的溶解度为0.29%,而水在其中的溶解度为0.13%。干燥纯净的1,1,2,2-四氯乙烷对金属的腐蚀性不强,但在潮湿空气中会逐渐分解放出腐蚀性强的氯化氢。
-
无空气、水分和光存在时,1,1,2,2-四氯乙烷是一种稳定的物质。然而,在与空气接触时,它会慢慢脱去氯化氢,生成三氯乙烯及微量的光气。在有水分的情况下,它会逐渐分解放出氯化氢。当与空气或氧气共存并经紫外光照射时,会产生二氯乙酰氯。1,1,2,2-四氯乙烷在沸水或水蒸气中用铁、铝、锌等金属处理时,可还原成1,2-二氯乙烯;常温下它不与氯反应,在紫外光照射下会氯化生成六氯乙烷。此外,在活性炭等催化剂存在下热裂或与石灰乳反应都会生成三氯乙烯,与强碱加热则会产生极易爆炸的二氯乙炔。
-
-
分解:Poisonous gases, including phosgene, chlorine, and hydrogen chloride are produced in fire.
-
粘度:0.00177 Pa.s at 20 °C
-
腐蚀性:Corrosive liquid
-
燃烧热:-8.3464X10+08 J/kmol
-
汽化热:45.71 kJ/mol at 25 °C
-
表面张力:34.72 dynes/cm at 20 °C
-
电离电位:11.10 eV
-
气味阈值:The odor threshold in air is 1.5 ppm.
-
折光率:Index of refraction: 1.49410 at 20 °C/D
-
相对蒸发率:0.65 (Butyl acetate = 1)
-
保留指数:877;888.4;922.1;884;882;888;895;900;882;888.7;876;879;895;886;884;892;892;905;887;876;876;876;888;910
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 1 ppm (7 mg/m3) [skin] (Chloroethanes)
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:100 ppm
-
危险品标志:T+,N
-
安全说明:S36/37,S38,S45,S61
-
危险类别码:R51/53,R26/27
-
WGK Germany:3
-
海关编码:2903 19 00
-
危险品运输编号:UN 1702 6.1/PG 2
-
危险类别:6.1
-
RTECS号:KI8575000
-
包装等级:II
-
危险标志:GHS06,GHS09
-
危险性描述:H310 + H330,H411
-
危险性防范说明:P260,P262,P280,P302 + P352 + P310,P304 + P340 + P310,P361 + P364
-
储存条件:储存注意事项: - 储存在阴凉、通风的库房中。 - 远离火源和热源。 - 保持容器密封。 - 应与氧化剂、碱类、活性金属粉末及食用化学品分开存放,避免混合储存。 - 储存区应配备泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 61556 |
| CAS: | 79-34-5 |
| 中文名称: | 1,1,2,2-四氯乙烷 |
| 英文名称: | 1,1,2,2-tetrachloroethane;acetylene tetrachloride |
| 别 名: | 四氯化乙炔;对称四氯乙烷 |
| 分子式: | C 2 H 2 Cl 4 ;CHCl 2 CHCl 2 |
| 分子量: | 167.86 |
| 熔 点: | -43.8℃ 沸点:146.4 |
| 密 度: | 相对密度(水=1)1.60 |
| 蒸汽压: | |
| 溶解性: | 微溶于水,溶于乙醇、乙醚等 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色重质液体,有氯仿样的气味 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作溶剂,用于有机合成 |
2.对环境的影响:
该物质对环境可能有危害,在地下水中有蓄积作用。在对人类重要食物链中,特别是在水生生物中发生蓄积。
一、健康危害
侵入途径:吸入、食入、经皮肤吸收。
健康危害:对中枢神经系统有麻醉作用和抑制作用,可引起肝、肾和心肌损害。短期吸入主要为粘膜刺激症状。急性及亚急性中毒主要为消化道和神经系统症状。可有食欲减退、呕吐、腹痛、肝大、腹水。长期吸入可引起无力、头痛、失眠、便秘或腹泻、肝功损害和多发性神经炎。
二、毒理学资料及环境行为
毒性:是氯代烃类中毒性较大的一种。
急性毒性:LD 50 800mg/kg(大鼠经口);LC 50 4500mg/m 3 ,2小时(小鼠吸入);人吸入1g/m×30分钟,呼吸道粘膜刺激,倦怠,眩晕,头沉;人吸入2~3g/m 3 ×10分钟,呼吸道粘膜刺激,倦怠,眩晕,头沉。
亚急性和慢性毒性:小鼠吸入47.9g/m 3 ×2小时/日×5日,死亡,肝脏损害;小鼠吸入3.0g/m 3 ×5~6小时/日×4日,死亡,心肌损害;大鼠吸入47.9g/m 3 ×2小时/日×5日,兴奋,肝损害,后期死亡。
致突变性:微生物致突变:鼠伤寒沙门氏菌200ul/皿微粒体致突变:鼠伤寒沙门氏菌200ul/皿。
致癌性:IARC致癌性评论:动物为可疑性反应。大鼠(osborne-mendel)经口43~108mg/kg/日×78周,出现肝癌及肿瘤结节。
危险特性:遇明火、高热可燃。受高热分解产生有毒的腐蚀性气体。与碱金属能发生剧烈反应。
燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢。
3.现场应急监测方法:
水质检测管法
4.实验室监测方法:
气相色谱法《固体废弃物试验与分析评价手册》中国环境监测总站等译
气相色谱法《城市和工业废水中有机化合物分析》王克欧等译
色谱/质谱法 美国EPA524.2方法
5.环境标准:
| 前苏联 | 车间空气中有害物质的最高容许浓度 | 5mg/m 3 |
| 前苏联(1978) | 生活饮用水和娱乐用水水体中有害物质的最大允许浓度 | 0.2mg/L |
| 前苏联(1975) | 污水排放标准 | 5mg/L |
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。不要直接接触泄漏物。尽可能切断泄漏源。防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。大量泄漏:构筑围堤或挖坑收容;用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
废弃物处置方法:用焚烧法。废料同其它燃料混合后焚烧,燃烧要充分,防止生成光气。焚烧炉排气中的卤化氢通过酸洗涤器除去。
二、防护措施
呼吸系统防护:空气中浓度超标时,应该佩戴直接式防毒面具(半面罩)。紧急事态抢救或撤离时,佩戴空气呼吸器。
眼睛防护:戴安全防护眼镜。
身体防护:穿防毒物渗透工作服。
手防护:戴防化学品手套。
其它;工作现场禁止吸烟、进食和饮水。工作毕,沐浴更衣。单独存放被毒物污染的衣服,洗后备用。注意个人清洁卫生。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量温水,催吐,就医。
灭火方法:雾状水、泡沫、二氧化碳、砂土。
制备方法与用途
1,1,2,2-四氯乙烷是一种乙烷的氯化衍生物,是溶解能力最强的有机氯化合物。作为制冷剂,它又被称为R-130。
理化性质1,1,2,2-四氯乙烷为无色、易流动的液体,具有甜味和强烈类似氯仿的气味。其熔点为-42.5℃,沸点为146.5℃,折射率为1.4942。它难溶于水,能与醇、醚、石油醚、卤代烃、二硫化碳等大多数有机溶剂混溶,是氯烃类中溶解能力最强的。
应用1,1,2,2-四氯乙烷主要被用作生产三氯乙烯和四氯乙烯的原料。此外,它还用于树脂、橡胶、脂肪等不易燃烧溶剂。
危害1,1,2,2-四氯乙烷在氯代烃类化合物中具有较大的毒性,可通过吸入、食入或经皮肤吸收三种途径导致人体中毒,对中枢神经系统及肝、肾和心肌造成损害。在人体内脂质过氧化是其导致肝损害的主要机制之一。
化学性质1,1,2,2-四氯乙烷为无色液体。熔点为-44℃,沸点为146.5℃(在6.0kPa下),相对密度为1.58658(25/4℃),折射率为1.49419。它能与乙醇、甲醇、乙醚、氯仿、苯、四氯化碳、二硫化碳、石油醚、二甲基甲酰胺及油类混溶,难溶于水。该物质还能随水蒸气挥发,并具有类似氯仿的气味,但不燃烧。
用途1,1,2,2-四氯乙烷是一种有效的溶剂,但由于其毒性较大,限制了其在某些方面的应用。工业中主要用作三氯乙烯和四氯乙烯的原料。此外,它还可用作溶剂及分析试剂、金属净洗剂、杀虫剂、除草剂等。
生产方法1,1,2,2-四氯乙烷由乙炔氯化制得。反应在四氯乙烷本身作为溶剂下进行(气态乙炔和氯直接反应会发生爆炸),催化剂为五氯化锑或三氯化铁。当采用三氯化铁催化剂时,需保持系统负压,并使四氯乙烷处于回流状态,连续通入干燥的乙炔和氯气,通过回流的四氯乙烷蒸气吸收反应热移走热量,收率为97%(对乙炔)。
类别 农药毒性分级:高毒
- 急性毒性:口服-大鼠LD50: 250毫克/公斤;腹腔-小鼠 LD50: 821毫克/公斤
不易燃,但接触氢氧化钾加热会放出易燃气体。水可促进分解,燃烧释放有毒氯化物烟雾
储运特性库房通风、低温干燥;与氧化剂、食品添加剂分开存放
灭火剂泡沫、干粉、砂土
职业标准TWA 35毫克/立方米; STEL 35毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1,2-三氯乙烷 1,1,2-trichloroethane 79-00-5 C2H3Cl3 133.405 五氯乙烷 pentachloroethane 76-01-7 C2HCl5 202.295 1,1-二氯乙烷 1,1-dichloroethane 75-34-3 C2H4Cl2 98.9598 六氯乙烷 hexachloroethane 67-72-1 C2Cl6 236.74 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 五氯乙烷 pentachloroethane 76-01-7 C2HCl5 202.295 1,1,1,2-四氯乙烷 1,1,1,2-tetrachoroethane 630-20-6 C2H2Cl4 167.85 六氯乙烷 hexachloroethane 67-72-1 C2Cl6 236.74
反应信息
-
作为反应物:描述:参考文献:名称:Jay, P., Chimie et Industrie (Paris), 1964, vol. 92, p. 533 - 537摘要:DOI:
-
作为产物:描述:参考文献:名称:Reduction of the CCl3 group in polychloroalkanes by hydride complexes of cyclopentadienyl derivatives of zirconium摘要:DOI:10.1007/bf01157366
-
作为试剂:描述:4-羟基香豆素 、 4-甲氧基苄醇 在 calcium chloride 、 1,1,2,2-四氯乙烷 作用下, 生成 4-hydroxy-3-(4-methoxybenzyl)-2H-chromen-2-one参考文献:名称:Zur Chemie des 4-Hydroxy-cumarins摘要:DOI:10.1007/bf01075426
文献信息
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
[EN] PYRROLOTRIAZINONE DERIVATIVES AS PI3K INHIBITORS<br/>[FR] DÉRIVÉS DE PYRROLOTRIAZINONE EN TANT QU'INHIBITEURS DES PI3K申请人:ALMIRALL SA公开号:WO2014060432A1公开(公告)日:2014-04-24New pyrrolotriazinone derivatives having the chemical structure of formula (I), are disclosed; as well as process for their preparation, pharmaceutical compositions comprising them and their use in therapy as inhibitors of Phosphoinositide 3-Kinases (PI3Ks)
-
Piperidyindoles as serotonin receptor ligands申请人:——公开号:US20030225068A1公开(公告)日:2003-12-04A pharmaceutical compound of the formula (I) in which R 1 and R 2 are each hydrogen or C 1-6 alkyl, R 3 is —SR 10 , —SOR 10 , —SO 2 R 10 , —COR 10 , —CH 2 OH or —CONHR 11 , where R 10 is C 1-6 alkyl and R 11 is hydrogen or C 1-6 alkyl, R 4 , R 5 , R 6 and R 7 are each hydrogen or C 1-6 alkyl, provided that at least one of R 4 , R 5 , R 6 and R 7 is C 1-6 alkyl, R 8 and R 9 are each hydrogen, halo, C 1-6 alkyl or cyano, n is 0 or 1 and m is 2 or 3, x is a (a) or (b), and y is (c) or (d), wherein R 12 and R 13 are each hydrogen, C 1- alkyl, cyclopropyl or cyclopropyl-C 1-6 alkyl; and salts thereof. 1
表征谱图
-
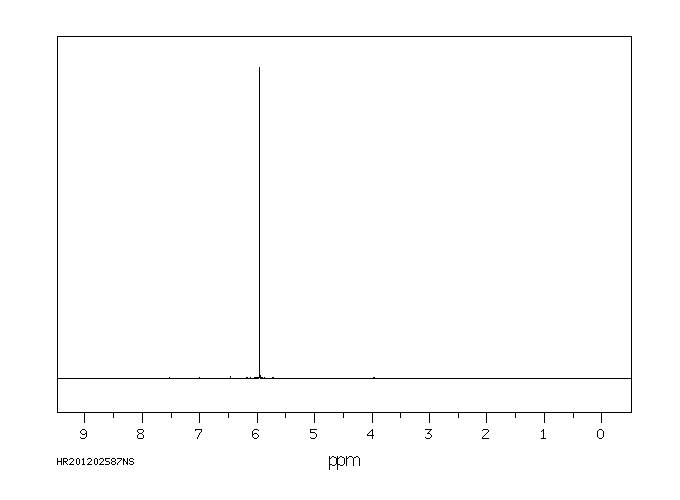
氢谱1HNMR
-
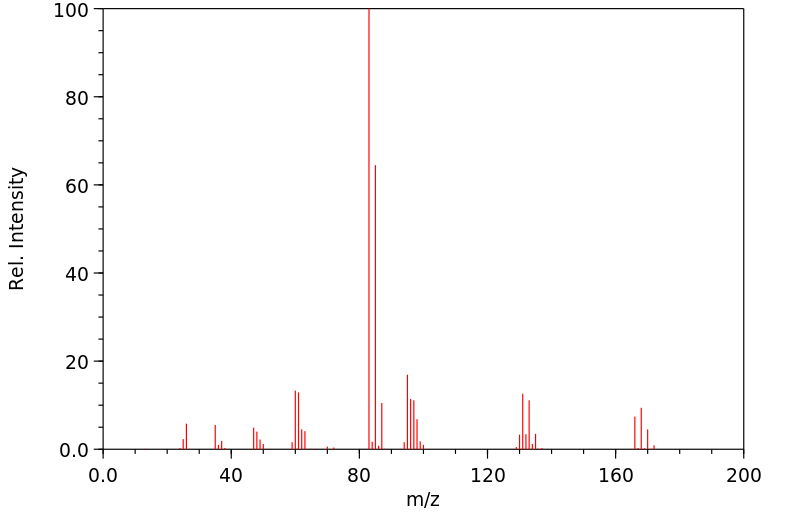
质谱MS
-
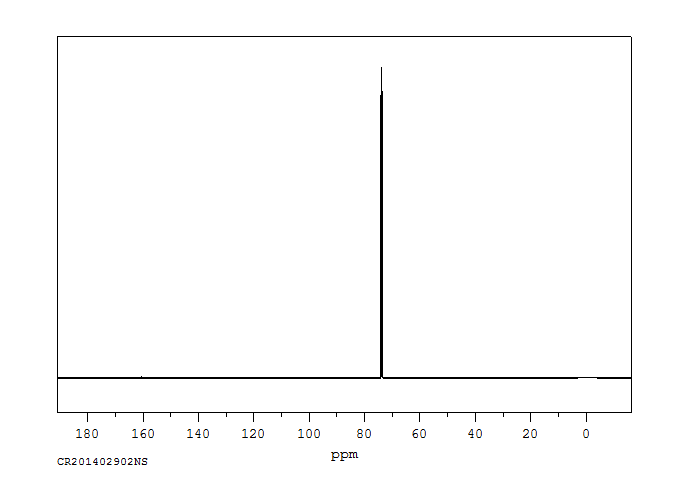
碳谱13CNMR
-
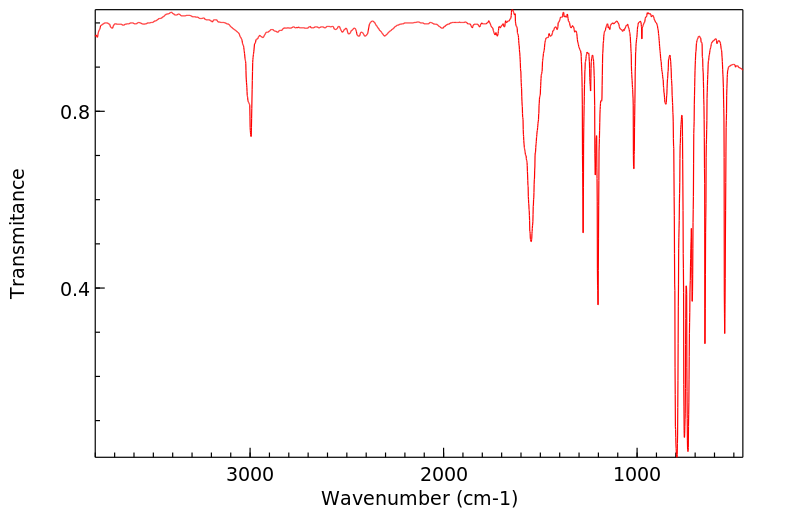
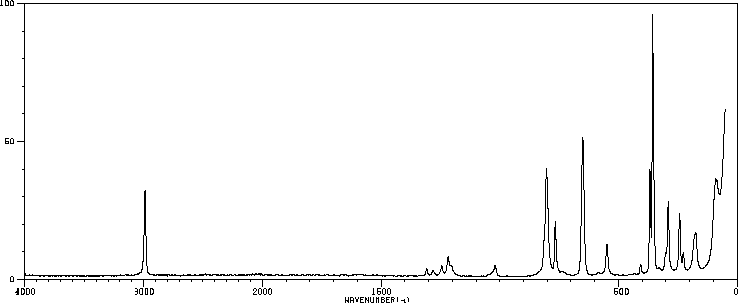
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息