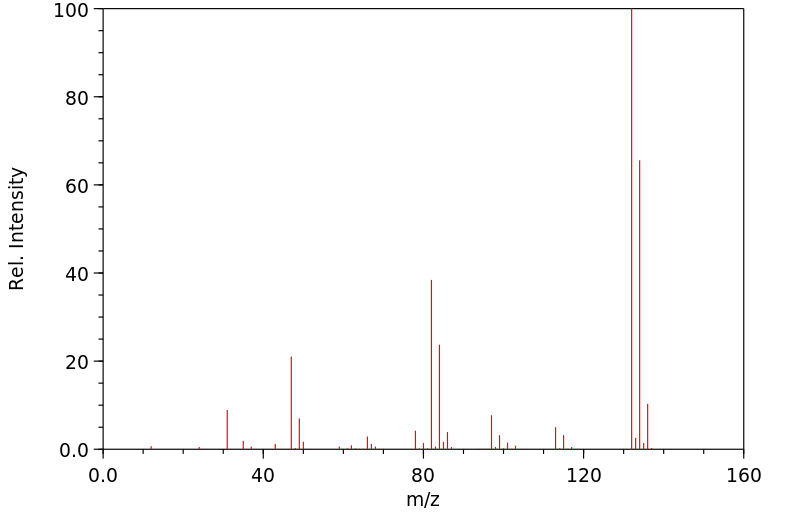
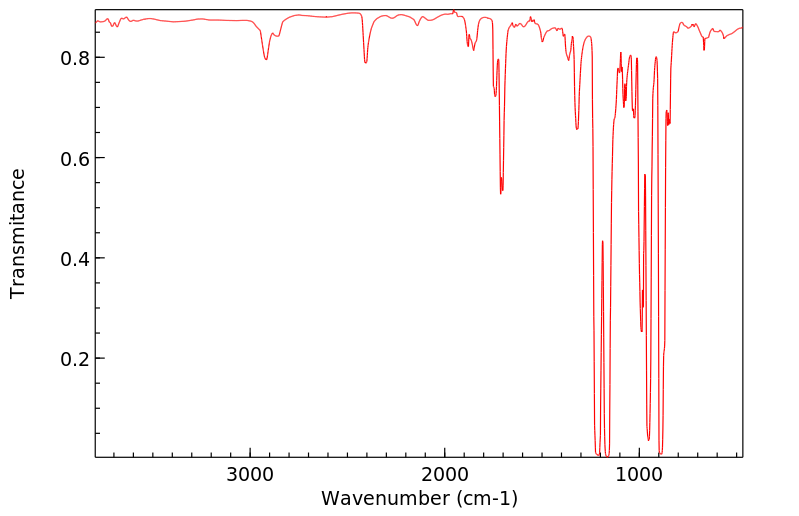
申请人:The Dow Chemical Company
公开号:US04349568A1
公开(公告)日:1982-09-14
Novel compounds exhibiting antiviral activity are disclosed, such compounds are represented by the formula: ##STR1## wherein m represents the integer 0, 1 or 2; R represents trihalomethyl, alkyl or phenyl; R.sub.1 represents hydrogen, bromo, chloro, fluoro, cyano, nitro, amino, alkyl, alkoxy or trifluoromethyl; R.sub.2 represents hydroxyl, bromo, chloro, fluoro, cyano, acetyl, benzoyl, substituted benzoyl, alkyl, alkoxy, substituted alkoxy, benzyl, substituted benzyl, alkylthio, alkylsulfinyl, alkylsulfonyl, alkylaminosulfonyl, dialkylaminosulfonyl, or the radical --O(CH.sub.2).sub.n R.sub.4 wherein n represents the integer 1, 2 or 3 and R.sub.4 represents cyano or dialkylamino; R.sub.3 represents hydrogen, hydroxyl, bromo, chloro, fluoro, cyano, acetyl, benzoyl, substituted benzoyl, alkyl, alkoxy, substituted alkoxy, benzyl, substituted benzyl, alkylthio, alkylsulfinyl, alkylsulfonyl, alkylaminosulfonyl, dialkylaminosulfonyl, or the radical --O(CH.sub.2).sub.n R.sub.4 wherein n represents the integer 1, 2 or 3; and R.sub.4 represents cyano or dialkylamino; or alternatively R.sub.2 and R.sub.3 taken together represent the radical --O--C(X).sub.2 --O-- wherein X represents hydrogen, bromo, chloro or fluoro. Methods of using the above-noted compounds and structurally related compounds to employ their antiviral activity are also disclosed as well as pharmaceutically-acceptable compositions.
披露了具有抗病毒活性的新型化合物,这些化合物可以用以下公式表示:##STR1## 其中,m代表整数0、1或2;R代表三卤甲基、烷基或苯基;R.sub.1代表氢、
溴、
氯、
氟、
氰、硝基、
氨基、烷基、烷氧基或三
氟甲基;R.sub.2代表羟基、
溴、
氯、
氟、
氰、乙酰基、苯甲酰基、取代的苯甲酰基、烷基、烷氧基、取代的烷氧基、苄基、取代的苄基、烷基亚砜、烷基亚磺酰基、烷基亚磺酰基、烷基
氨磺酰基、二烷基
氨磺酰基,或者自由基--O(CH.sub.2).sub.n R.sub.4,其中n代表整数1、2或3,R.sub.4代表
氰或二烷基
氨基;R.sub.3代表氢、羟基、
溴、
氯、
氟、
氰、乙酰基、苯甲酰基、取代的苯甲酰基、烷基、烷氧基、取代的烷氧基、苄基、取代的苄基、烷基亚砜、烷基亚磺酰基、烷基亚磺酰基、烷基
氨磺酰基、二烷基
氨磺酰基,或者自由基--O(CH.sub.2).sub.n R.sub.4,其中n代表整数1、2或3;R.sub.4代表
氰或二烷基
氨基;或者R.sub.2和R.sub.3共同代表自由基--O--C(X).sub.2 --O--,其中X代表氢、
溴、
氯或
氟。还披露了使用上述化合物的结构相关化合物来发挥它们的抗病毒活性的方法,以及药物可接受的组合物。