代谢
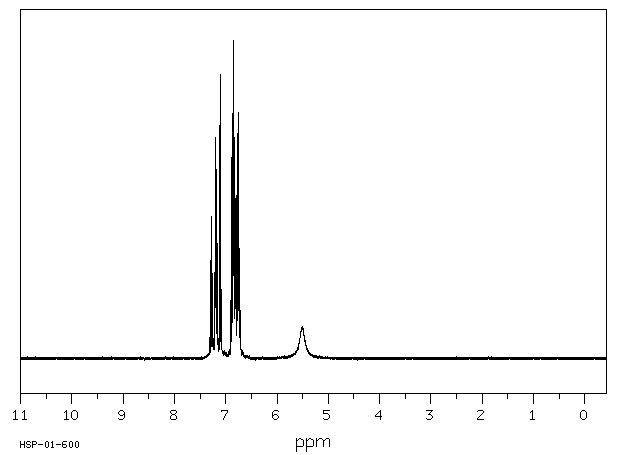
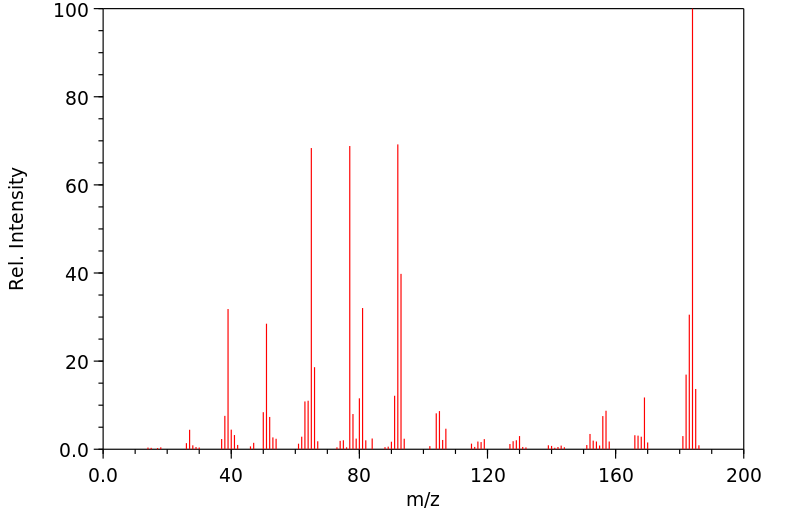
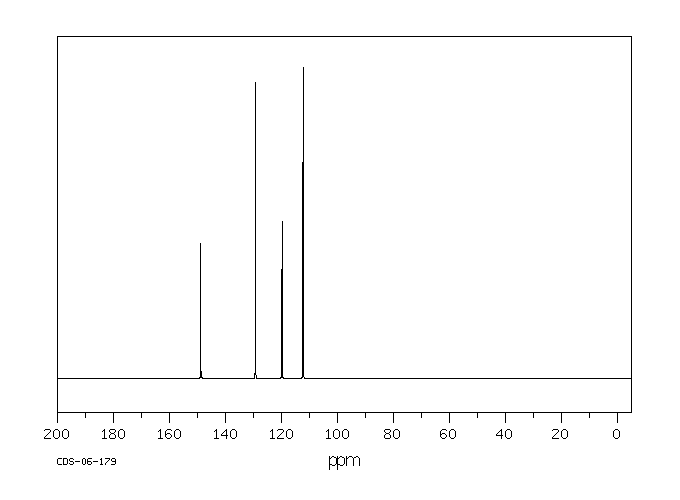
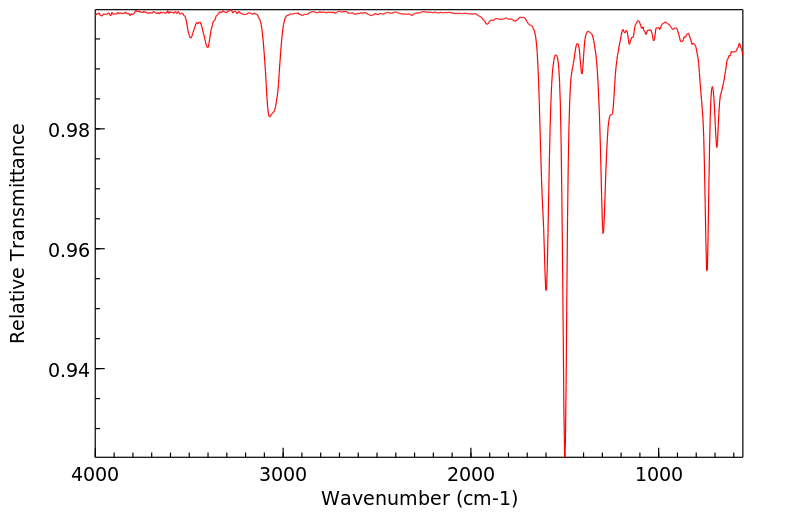
对给予200或400 mg/kg口服剂量的老鼠的尿液进行薄层色谱分析,发现了联苯胺、苯肼和对苯二胺的存在,以及另外两种未识别的代谢物。在100或200 mg/kg的ip剂量下,尿液中含有联苯胺、苯胺、对苯二胺、对氨基酚和邻氨基酚。静脉注射或气管内给药后,在尿液中仅发现一种代谢物,似乎具有酚类特性。
Thin layer chromatographic analysis of the urine of rats given an oral dose of 200 or 400 mg/kg revealed the presence of hydrazobenzene, benzidine, and two other unidentified metabolites. At an ip dose of 100 or 200 mg/kg, hydrazobenzene, aniline, benzidine, p-aminophenol, and o-aminophenol were identified in the urine. After iv or intratracheal administration of hydrazobenzene, only one urinary metabolite, which appeared to be phenolic, was found.
来源:Hazardous Substances Data Bank (HSDB)