1,3,5-三苯酰基苯 | 25871-69-6
中文名称
1,3,5-三苯酰基苯
中文别名
1,3,5-三苯甲酰基苯
英文名称
1,3,5-tribenzoylbenzene
英文别名
benzene-1,3,5-triyltris(phenylmethanone);1,3,5-Tribenzoylbenzol;1,3,5-tris(α-benzoyl)benzene;Tribenzoylbenzol;(3,5-dibenzoylphenyl)-phenylmethanone
CAS
25871-69-6
化学式
C27H18O3
mdl
——
分子量
390.438
InChiKey
FDSRSUAVHPFWGT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118-119°C
-
沸点:475.65°C (rough estimate)
-
密度:1.1256 (rough estimate)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:30
-
可旋转键数:6
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
海关编码:2914399090
-
安全说明:S24/25
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:保持贮藏器密封,并将其放入一个紧密的容器中。储存在阴凉、干燥的地方。
SDS
制备方法与用途
1,3,5-三苯酰基苯用于作为研究用化合物。
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:利用多功能引发剂的定义明确,规则支化的聚苯乙烯的合成与表征摘要:通过阴离子聚合合成了一系列结构明确的长支链聚苯乙烯(PS),但总分子量相同,适合系统地研究支化效应,并进行了表征。用三官能的有机锂引发剂合成了三个具有6、9和13个端支链的端支,星形支化聚苯乙烯。13末端分子的合成需要最近开发的甲氧基甲硅烷基官能化和沉淀程序,以去除多余的连接剂。在这些体系结构中,分支点的数量固定为四个,而链端的数量则变化。用双官能有机锂引发剂合成了具有两个分支点的六端绒球(哑铃形)PS。包括具有一个分支点的常规六臂星形聚苯乙烯,以提供三种聚合物的比较,每种聚合物具有6个末端,但分支点的数量等于1、2或4。本征粘度和无限稀释扩散系数(因此分支因子和流体动力学半径)随链端数目的增加而减小,但不会随分支点数目的变化而单调变化。的值分子的T g既反映了连接点的束缚效应,又由于链端的倍增以及用于促进连接的丁二烯单元的存在而增加了自由体积。DOI:10.1021/ma050207i
-
作为产物:描述:(E)-beta-氯丙烯酰苯 反应 360.0h, 生成 1,3,5-三苯酰基苯参考文献:名称:Bhaskar Reddy; Chandrasekhar Babu; Venugopal Reddy, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2001, vol. 40, # 5, p. 416 - 418摘要:DOI:
文献信息
-
Microporous, tetraarylethylene-based polymer networks generated in a reductive polyolefination process作者:E. Preis、W. Dong、G. Brunklaus、U. ScherfDOI:10.1039/c4tc02664k日期:——
Highly fluorescent microporous polymer networks with triazine cores have been prepared and tested for their PL quenching response to arylamines.
高荧光三嗪核微孔聚合物网络已经制备并测试其对芳胺的荧光猝灭响应。 -
芳香酮化合物及其有机发光器件
-
Palladium-Promoted Transformation of β-Amino Ketones to Enaminones作者:Shun-Ichi Murahashi、Yo Mitsue、Tatsuo TsumiyamaDOI:10.1246/bcsj.60.3285日期:1987.9The reaction of β-amino ketones with bis(acetonitrile)dichloropalladium(II) in the presence of triethylamine gives the corresponding enaminones regioselectively. The cyclic β-amino ketones can be converted into the corresponding exocyclic enaminones. The enaminones thus obtained are versatile synthetic intermediates. The reaction of (E)-enaminones with organocuprates gave the corresponding (E)-α,β-unsaturated ketones.
-
Iron-Catalyzed Trimerization of Terminal Alkynes Enabled by Pyrimidinediimine Ligands: A Regioselective Method for the Synthesis of 1,3,5-Substituted Arenes作者:Julianna S. Doll、Robert Eichelmann、Leif E. Hertwig、Thilo Bender、Vincenz J. Kohler、Eckhard Bill、Hubert Wadepohl、Dragoş-Adrian RoşcaDOI:10.1021/acscatal.1c00978日期:2021.5.7to a functional-group-tolerant methodology for the catalytic trimerization of terminal aliphatic alkynes. Remarkably, in contrast to established alkyne trimerization protocols, the 1,3,5-substituted arenes are the main reaction products. Preliminary mechanistic investigations suggest that the enhanced π-acidity of the pyrimidine ring, combined with the hemilability of the imine groups coordinated to
-
Enantioselective construction of 2,5-dihydropyrrole skeleton with quaternary stereogenic center via catalytic asymmetric 1,3-dipolar cycloaddition involving α-arylglycine esters作者:Feng Shi、Gui-Juan Xing、Wei Tan、Ren-Yi Zhu、Shujiang TuDOI:10.1039/c2ob26566d日期:——A catalytic asymmetric construction of synthetically and biologically important 2,5-dihydropyrrole scaffolds with concomitant creation of multiple chiral carbon centers including one quaternary stereogenic center in high yields (up to 99%) and excellent enantioselectivities (up to 99% ee) has been established via an organocatalytic 1,3-dipolar cycloaddition using α-arylglycine esters as azomethine precursors
表征谱图
-
氢谱1HNMR
-
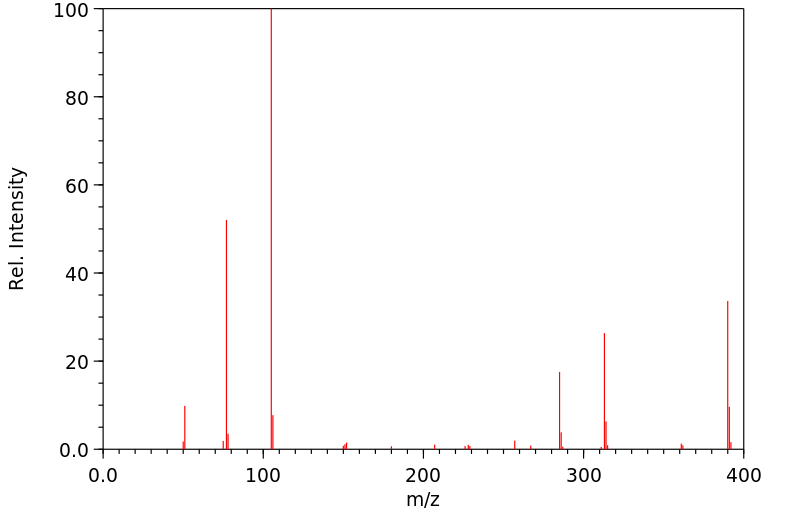
质谱MS
-
碳谱13CNMR
-
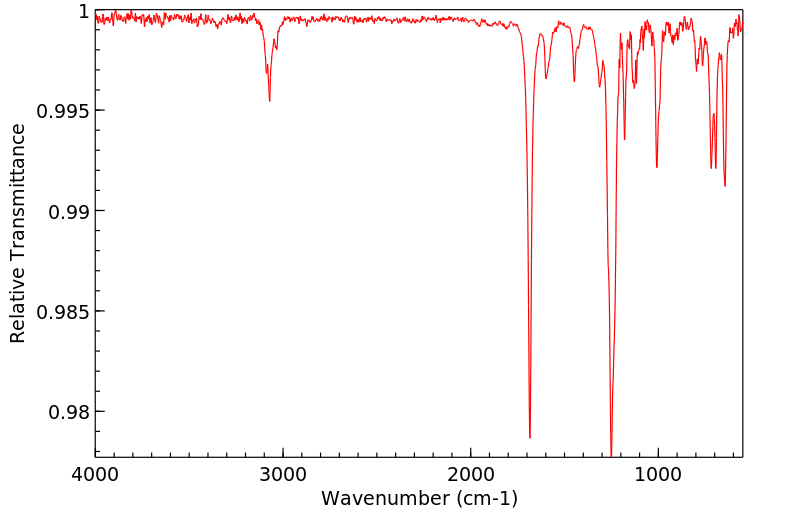
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11aR)-3,7-双(3,5-二甲基苯基)-10,11,12,13-四氢-5-羟基-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂膦酸
龙血素C
顺-1,7-二苯基-1-庚烯基-5-醇
那洛西芬
赤杨酮
赤杨二醇
血竭素
蒙桑酮C
萘-2,7-二磺基酸,钠盐
苯酚,4-(1,3-二苯基丁基)-2-(1-苯基乙基)-
苯甲酸,2-[[2-[(2-羧基苯基)氨基]-5-(三氟甲基)苯基]氨基]-5-[[[(4-羟基-3-甲氧苯基)甲基]氨基]甲基]-
苯基-[4-(2-苯基乙炔基)苯基]甲酮
苯基-[2-[3-(三氟甲基)苯基]苯基]甲酮
苯基-[2-(2-苯基苯基)苯基]甲酮
苯基-(3-苯基萘-2-基)甲酮
苯基-(2-苯基环己基)甲酮
苯,[(二甲基苯基)甲基]甲基[(甲基苯基)甲基]-
苯,1,3-二[1-甲基-1-[4-(4-硝基苯氧基)苯基]乙基]-
脱甲氧姜黄
紫外吸收剂 234
粗糠柴苦素
硫酸姜黄素
矮紫玉盘素
益智醇
白桦林烯酮;1,7-双(4-羟基苯基)-4-庚烯-3-酮
甲酮,苯基(1,6,7,8-四氢-1-甲基-5-苯基环戊二烯并[g]吲哚-3-基)-
甲酮,[3-(4-甲氧苯基)-1-苯基-9H-芴-4-基]苯基-
甲酮,(4-氯苯基)[1-(4-氯苯基)-3-苯基-9H-芴-4-基]-
环香草酮
溴敌隆
波森
桤木酮
桑根酮D
杨梅醇
杨梅酮
杨梅联苯环庚醇-15-葡糖苷
替拉那韦
替吡法尼(S型对映体)
替吡法尼
曲沃昔芬
姜黄素葡糖苷酸
姜黄素beta-D-葡糖苷酸
姜黄素4,4'-二乙酸酯
姜黄素-d6
姜黄素
姜烯酮 A
奈帕芬胺杂质D
四甲基姜黄素
四氢脱甲氧基二阿魏酰甲烷
四氢姜黄素二乙酸酯