1-碘双环[2.2.2]辛烷 | 931-98-6
中文名称
1-碘双环[2.2.2]辛烷
中文别名
——
英文名称
1-Iodobicyclo<2.2.2>octane
英文别名
1-I-bicyclo<2.2.2>octane;1-iodobicyclo[2.2.2]octane;1-iodobicyclo{2.2.2}octane;1-Iod-bicyclo<2.2.2>octan;1-Jod-bicyclo<2.2.2>octan;1-Jodobicyclo<2.2.2>octan
CAS
931-98-6
化学式
C8H13I
mdl
——
分子量
236.096
InChiKey
SMDJHIPXLFNPTJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:9
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-二碘双环[2.2.2]辛烷 1,4-diiodobicyclo<2.2.2>octane 10364-05-3 C8H12I2 361.992 —— 1-Hydroxy-4-iodobicyclo<2.2.2>octane 74467-17-7 C8H13IO 252.095
反应信息
-
作为反应物:描述:参考文献:名称:Reactions of some bicycloalkyl iodides with bromine摘要:DOI:10.1021/jo00135a007
-
作为产物:描述:1,4-二氯双环[2.2.2]辛烷 在 氢氧化钾 、 aluminum tri-bromide 、 氢碘酸 、 叔丁基锂 、 silver(I) acetate 、 碘乙烷 作用下, 以 甲醇 、 乙醚 、 溶剂黄146 、 正戊烷 为溶剂, 反应 6.0h, 生成 1-碘双环[2.2.2]辛烷参考文献:名称:Rod-like organic molecules. Energy-transfer studies using single-photon counting摘要:DOI:10.1021/jo01308a001
文献信息
-
Photoinduced Copper‐Catalyzed Coupling of Terminal Alkynes and Alkyl Iodides作者:Avijit Hazra、Mitchell T. Lee、Justin F. Chiu、Gojko LalicDOI:10.1002/anie.201801085日期:2018.5.4We have developed a photoinduced copper‐catalyzed alkylation of terminal alkynes with primary, secondary, or tertiary alkyl iodides as electrophiles. The reaction has a broad substrate scope and can be successfully performed in the presence of ester, nitrile, aryl halide, ketone, sulfonamide, epoxide, alcohol, and amide functional groups. The alkylation is promoted by blue light (λ≈450 nm) and proceeds
-
Photochemistry of alkyl halides. 4. 1-Norbornyl, 1-norbornylmethyl, 1- and 2-adamantyl, and 1-octyl bromides and iodides作者:Paul J. Kropp、Graham S. Poindexter、Norbert J. Pienta、David C. HamiltonDOI:10.1021/ja00441a043日期:1976.12Competing ionic and radical photobehavior has been observed for a number of alkyl halides. The proportion of nucleophilic substitution and reduction products were reported for each halide. Solvent effects of ethylene glycol, triethylamine, and methanol and atmospheric effects of nitrogen, oxygen, and air were studied. The effect of irradiation of the halides with methanol-O-d was reported. The results
-
Synthesis of bridgehead fluorides by fluorodeiodination作者:Ernest W. Della、Nicholas J. HeadDOI:10.1021/jo00036a018日期:1992.5Fluorodeiodination is found to be an attractive procedure for the synthesis of bridgehead fluorides. Thus, treatment of the corresponding iodide with xenon difluoride in dichloromethane at ambient temperature generally leads to high yields of the fluoride. Evidence suggests the intermediacy of the bridgehead cation in this reaction, and accordingly the substrates which are unfavorably disposed to fluorodeiodination are the bicyclo[n.1.1]alkyl iodides. In this context the isolation of a small quantity of methyl 4-fluorobicyclo[2.1.1]hexane-1-carboxylate (46, R = COOMe) is significant because it represents the first occasion on which the elusive 1-bicyclo[2.1.1]hexyl cation has been trapped. We have also demonstrated that synthesis of the iodides themselves can be accomplished efficiently both by Barton halodecarboxylation and by treatment of the carboxylic acid with lead tetraacetate and iodine.
-
Kopecky, Jan; Smejkal, Jaroslav, Collection of Czechoslovak Chemical Communications, 1980, vol. 45, # 11, p. 2965 - 2970作者:Kopecky, Jan、Smejkal, JaroslavDOI:——日期:——
-
Bridged ring compounds. VIII. 1-Halobicyclo[2.2.-2]octanes作者:Zennosuke Suzuki、Kenichi MoritaDOI:10.1021/jo01277a008日期:1967.1
表征谱图
-
氢谱1HNMR
-
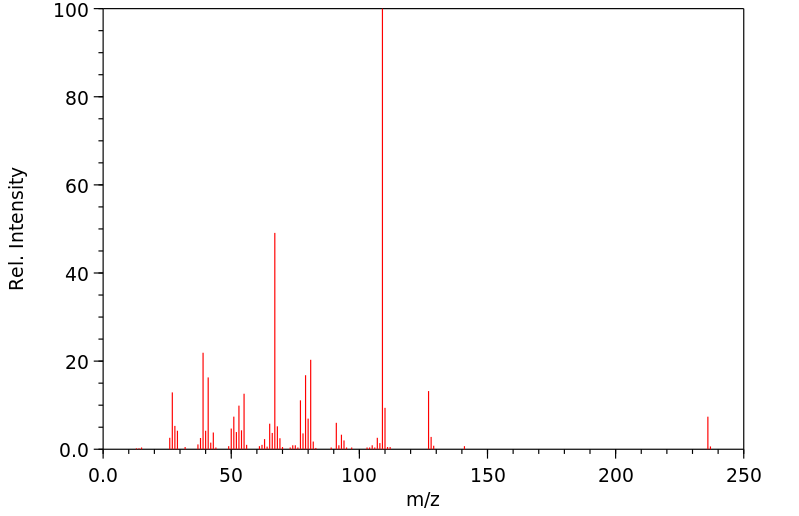
质谱MS
-
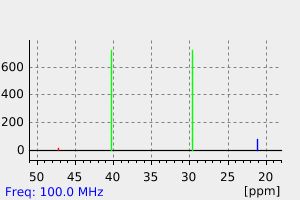
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物