2 - 甲氧基喹啉 | 6931-16-4
中文名称
2 - 甲氧基喹啉
中文别名
2-甲氧基喹啉
英文名称
2-methoxy-quinoline
英文别名
2-Methoxyquinoline
CAS
6931-16-4
化学式
C10H9NO
mdl
MFCD01070847
分子量
159.188
InChiKey
ZTQNUTNKGQGWCM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:219-220℃
-
沸点:60-63℃ (0.13 Torr)
-
保留指数:1377;235.35
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:22.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933499090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Methoxyquinoline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Methoxyquinoline
CAS number: 6931-16-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H9NO
Molecular weight: 159.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Methoxyquinoline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Methoxyquinoline
CAS number: 6931-16-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H9NO
Molecular weight: 159.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-喹啉醇 2-quinolone 70254-42-1 C9H7NO 145.161 4-氯-2-甲氧基喹啉 2-methoxy-4-chloroquinoline 4295-05-0 C10H8ClNO 193.633 喹啉 quinoline 91-22-5 C9H7N 129.161 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-喹啉醇 2-quinolone 70254-42-1 C9H7NO 145.161 6-溴-2-甲氧基喹啉 6-bromo-2-methoxyquinoline 99455-05-7 C10H8BrNO 238.084 —— 2-(neopentyloxy)quinoline —— C14H17NO 215.295 —— 2-methoxyquinoline 1-oxide 10285-99-1 C10H9NO2 175.187 8-溴-2-甲氧基喹啉 8-bromo-2-methoxyquinoline 146564-18-3 C10H8BrNO 238.084 —— 2-methoxy-6-nitroquinoline 116529-85-2 C10H8N2O3 204.185 2-甲氧基喹啉-3-硼酸 (2-methoxyquinolin-3-yl)boronic acid 886853-93-6 C10H10BNO3 203.005
反应信息
-
作为反应物:描述:2 - 甲氧基喹啉 600.0 ℃ 、1.33 Pa 条件下, 生成 1-甲基-2-喹啉酮参考文献:名称:2-烷氧基吡啶热诱导重排为 N-烷基吡啶酮摘要:2-甲氧基吡啶的类似物在快速真空热解 (FVP) 条件下重排为 N-甲基吡啶酮。乙氧基衍生物经历竞争性乙基迁移和乙烯消除。4-甲氧基吡啶的类似物在 FVP 条件下不会发生重排,但在二氧化硅上可能会发生去甲基化。重排的容易程度在一定程度上遵循烷氧基杂环的碱性。气相重排与凝聚相热解形成对比,计算支持前者的四中心过渡态。重排允许对来自 2,4-二氯喹啉与吡咯烷反应的两种产物进行结构分配。DOI:10.1071/ch03044
-
作为产物:描述:2,2'-oxybis(4-chloroquinoline) 在 palladium on activated charcoal 氢气 、 sodium methylate 、 sodium acetate 作用下, 以 甲醇 为溶剂, 反应 6.0h, 生成 2 - 甲氧基喹啉参考文献:名称:Studies on tertiary amine oxides. LXVI. Reactions of quinoline 1-oxide derivatives with tosyl chloride in the presence of triethylamine.摘要:脂肪噻嗪氧化物(1a)与4-甲基(1b)、4-氯(1c)和6-甲氧基喹啉(1d)在室温下与对甲苯磺酰氯(1当量)和三乙胺(约10当量)在氯仿和水的混合物中反应,得到了相应的二(2-喹啉基)醚(3a-d)和N-(2-喹啉基)-2-喹啉酮(4a-d)。这类反应的效率依赖于取代基的性质和位置。尽管在相同条件下,1c与碳基乙烯基的反应仅得到少量的4-氯-2-对甲苯磺酰氧基喹啉(2c)和N-(4-氯-2-喹啉基)-4-氯-2-喹啉酮(4c),但1d的反应则分别获得了6-甲氧基-2-喹啉基-2'-喹啉醚(15)和N-(6-甲氧基-2-喹啉基)-2-喹啉酮(16),产率为47%和29%。DOI:10.1248/cpb.28.493
-
作为试剂:参考文献:名称:具有单离子磁行为的单核Dy配合物及其对1,4-二恶烷的高选择性检测摘要:摘要用有机配体5,7-二溴-2-甲基-8-喹啉(L)在60°C下与Dy(NO3)3·6H2O反应,得到配合物[Dy(L)3(CH3CH2OH)( H 2 O)]·CH 3 CN(1)。此外,电喷雾质谱(ESI–MS)数据显示,在0 eV电压条件下,主要分子片段为[DyL2(DMF)2] +。当复合物1溶解在DMF中并停留了半个月时,在测量ESI-MS后它也显示出主要分子片段。因此,复合物1在DMF中相对稳定。磁化率表明1表现出清晰的场致SIM行为,在1000 Oe dc场下,能垒和外推弛豫时间分别为Ueff = 14.6 K和τ0= 4.1×10-5 s。将络合物1溶解在各种有机溶剂中并测试荧光。发现在1中具有很高的选择性DOI:10.1016/j.poly.2019.01.038
文献信息
-
Modulators of the Cystic Fibrosis Transmembrane Conductance Regulator Protein and Methods of Use申请人:AbbVie S.à.r.l.公开号:US20190077784A1公开(公告)日:2019-03-14The invention discloses compounds of Formula (I), wherein A 1 , R 1 , R 2 , R 3 , R 4 , and n are as defined herein. The present invention relates to compounds and their use in the treatment of cystic fibrosis, methods for their production, pharmaceutical compositions comprising the same, and methods of treating cystic fibrosis by administering a compound of the invention.该发明揭示了式(I)的化合物, 其中A 1 ,R 1 ,R 2 ,R 3 ,R 4 和n如本文所定义。本发明涉及化合物及其在囊性纤维化治疗中的应用,其生产方法,包含相同化合物的药物组合物,以及通过给予该发明的化合物来治疗囊性纤维化的方法。
-
Remote site-selective C–H activation directed by a catalytic bifunctional template作者:Zhipeng Zhang、Keita Tanaka、Jin-Quan YuDOI:10.1038/nature21418日期:2017.3In chemical syntheses, the activation of carbon–hydrogen (C–H) bonds converts them directly into carbon–carbon or carbon–heteroatom bonds without requiring any prior functionalization. C–H activation can thus substantially reduce the number of steps involved in a synthesis. A single specific C–H bond in a substrate can be activated by using a ‘directing’ (usually a functional) group to obtain the desired在化学合成中,碳-氢 (C-H) 键的活化将它们直接转化为碳-碳或碳-杂原子键,无需任何事先的功能化。因此,C-H 活化可以大大减少合成中涉及的步骤数。可以通过使用“定向”(通常是官能团)基团来激活底物中的单个特定 C-H 键,从而选择性地获得所需的产物。由于所讨论的 C-H 键与导向基团的距离以及底物的形状,这种 C-H 活化反应的适用性会受到严重限制,但已经开发了几种方法来克服这些限制。在这样一种方法中,通过使用共价连接的 U 形模板,已经利用对官能团和底物 C-H 键之间的远端和几何关系的理解来实现元选择性 C-H 激活。然而,在没有合适的官能团来连接它的情况下,这个模板的化学计量安装是不可行的。在这里,我们报告了一种催化双功能腈模板的设计,该模板通过可逆配位而不是共价键结合杂环底物。与该模板协调的两个金属中心具有不同的作用:一个可逆地锚定催化剂附近的底物,另一个切割远程 C-H 键。使用这种策略,我们演示了远程、
-
[EN] CYCLOHEXYL-AZETIDINYL ANTAGONISTS OF CCR2<br/>[FR] ANTAGONISTES DU CCR2 À BASE DE CYCLOHEXYL-AZÉTIDINYLE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2011159854A1公开(公告)日:2011-12-22The present invention comprises compounds of Formula (I). Wherein: R1, R2, R4, J, Q, and A are as defined in the specification. The invention also comprises a method of preventing, treating or ameliorating a syndrome, disorder or disease, wherein said syndrome, disorder or disease is type II diabetes, obesity and asthma. The invention also comprises a method of inhibiting CCR2 activity in a mammal by administration of a therapeutically effective amount of at least one compound of Formula (I).本发明包括公式(I)的化合物。其中:R1、R2、R4、J、Q和A如说明书所述定义。本发明还包括预防、治疗或改善综合征、障碍或疾病的方法,其中所述综合征、障碍或疾病为2型糖尿病、肥胖和哮喘。本发明还包括通过管理治疗有效量的至少一种公式(I)化合物来抑制哺乳动物中的CCR2活性的方法。
-
[EN] GTPASE INHIBITORS AND METHODS OF USE<br/>[FR] INHIBITEURS DE GTPASE ET PROCEDES D'UTILISATION CORRESPONDANTS申请人:CHILDRENS HOSP MEDICAL CENTER公开号:WO2005051392A1公开(公告)日:2005-06-09The preferred embodiments generally relate to methods and compositions that affect the GTP-binding activity of members of the Rho family GTPases, preferably Rac (Rac1, Rac2 and/or Rac3), such compositions include compounds that modulate the GTP/GDP exchange activity, along with uses for the compounds including screening for compounds which recognize Rac GTPase, and methods of treating pathological conditions associated or related to a Rho family GTPase, including Rac. The preferred embodiments also relate to methods of using such compounds, or derivatives thereof, e.g., in therapeutics, diagnostics, and as research tools.首选实施例通常涉及影响Rho家族GTP酶成员的GTP结合活性的方法和组合物,最好是Rac(Rac1、Rac2和/或Rac3),这些组合物包括调节GTP/GDP交换活性的化合物,以及这些化合物的用途,包括筛选识别Rac GTP酶的化合物,并用于治疗与Rho家族GTP酶(包括Rac)相关或相关的病理条件的方法。首选实施例还涉及使用这些化合物或其衍生物的方法,例如在治疗学、诊断学和作为研究工具中的用途。
-
GTPase inhibitors and methods of use and crystal structure of RAC-1 GTPase申请人:Zheng Yi公开号:US20070155766A1公开(公告)日:2007-07-05The preferred embodiments generally relate to methods and compositions that affect the GTP-binding activity of members of the Rho family GTPases, preferably Rac (Rac1, Rac2 and/or Rac3), such compositions include compounds that modulate the GTP/GDP exchange activity, along with uses for the compounds including screening for compounds which recognize Rac GTPase, and methods of treating pathological conditions associated or related to a Rho family GTPase, including Rac. The preferred embodiments also relate to methods of using such compounds, or derivatives thereof, e.g., in therapeutics, diagnostics, and as research tools.首选实施例通常涉及影响Rho家族GTP酶成员的GTP结合活性的方法和组合物,优选是Rac(Rac1、Rac2和/或Rac3),这些组合物包括调节GTP/GDP交换活性的化合物,以及这些化合物的用途,包括筛选识别Rac GTP酶的化合物,并用于治疗与Rho家族GTP酶(包括Rac)相关或相关的病理条件的方法。首选实施例还涉及使用这些化合物或其衍生物的方法,例如在治疗学、诊断学和作为研究工具中。
表征谱图
-
氢谱1HNMR
-
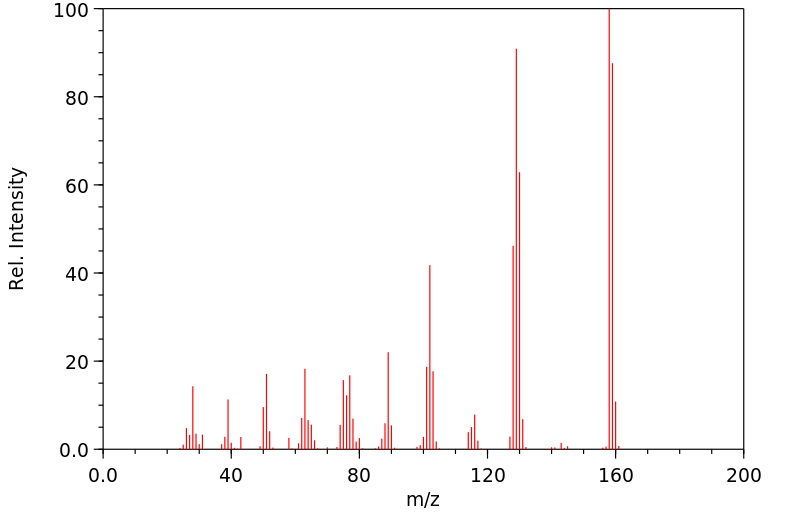
质谱MS
-
碳谱13CNMR
-
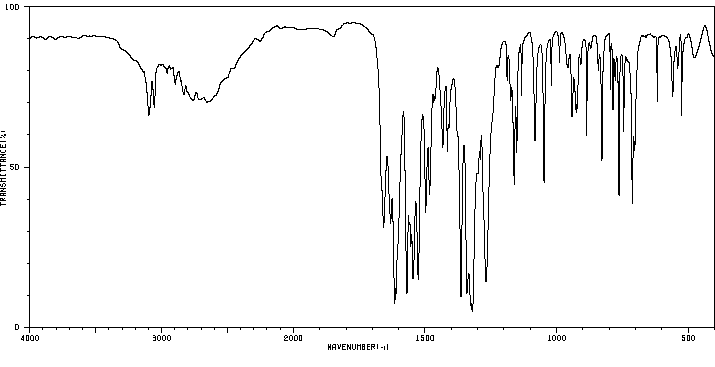
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43