2,4-二苯基吡咯 | 3274-56-4
中文名称
2,4-二苯基吡咯
中文别名
——
英文名称
2,4-diphenylpyrrole
英文别名
2,4-diphenyl-1H-pyrrole
CAS
3274-56-4
化学式
C16H13N
mdl
——
分子量
219.286
InChiKey
FSBPQTRUHOMNPG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:179-180 °C(Solv: benzene (71-43-2))
-
沸点:416.4±24.0 °C(Predicted)
-
密度:1.105±0.06 g/cm3(Predicted)
-
保留指数:1790;1790
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:17
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:15.8
-
氢给体数:1
-
氢受体数:0
安全信息
-
海关编码:2933990090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302,H317
-
储存条件:室温
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:A Modular Synthesis of Unsymmetrical Tetraarylazadipyrromethenes摘要:A stepwise route to unsymmetrical tetraarylazadipyrromethenes by a condensation of 2,4-diaryl-5-nitroso-pyrroles with 2,4-diarylpyrroles is described. This modular building-block approach allows for the introduction of up to four different aryl substituents on the azadipyrromethene and is tolerant of a varied substituent set. An efficient synthesis of the 2,4-diarylpyrroles building blocks from 1,3-diaryl-4-nitro-butan-1-ones by nitro hydrolysis to a keto-aldehyde and subsequent ammonia condensation reaction has been achieved. The facile conversion of 2,4-diarylpyrroles into their alpha-nitroso analogues by their reaction with sodium nitrite generated the second building block required for the synthesis.DOI:10.1021/jo050696k
-
作为产物:描述:4,4-dimethoxy-1,3-diphenyl-butan-1-one 在 ammonium acetate 、 溶剂黄146 作用下, 反应 1.0h, 以0.31 g的产率得到2,4-二苯基吡咯参考文献:名称:A Modular Synthesis of Unsymmetrical Tetraarylazadipyrromethenes摘要:A stepwise route to unsymmetrical tetraarylazadipyrromethenes by a condensation of 2,4-diaryl-5-nitroso-pyrroles with 2,4-diarylpyrroles is described. This modular building-block approach allows for the introduction of up to four different aryl substituents on the azadipyrromethene and is tolerant of a varied substituent set. An efficient synthesis of the 2,4-diarylpyrroles building blocks from 1,3-diaryl-4-nitro-butan-1-ones by nitro hydrolysis to a keto-aldehyde and subsequent ammonia condensation reaction has been achieved. The facile conversion of 2,4-diarylpyrroles into their alpha-nitroso analogues by their reaction with sodium nitrite generated the second building block required for the synthesis.DOI:10.1021/jo050696k
文献信息
-
NOVEL TYPE OF TRANSFORMATIONS OF α-AZIDOSTYRENE DERIVATIVES AND 3-ARYL-2<i>H</i>-AZIRINES IN THE PRESENCE OF HEXACARBONYLMOLYBDENUM作者:Makoto Nitta、Tomoshige KobayashiDOI:10.1246/cl.1983.1715日期:1983.11.5The reaction of α-azidostyrene derivatives with hexacarbonylmolybdenum was found to give 2,5-diarylpyrroles and acetophenone derivatives via a complexed 1-arylvinylnitrene intermediate, while that of 3-aryl-2H-azirines gave 2,4-diarylpyrroles in addition to acetophenone derivatives and 2,5-diarylpyrazines.
-
Efficient Far-Red/Near-IR Absorbing BODIPY Photocages by Blocking Unproductive Conical Intersections作者:Pradeep Shrestha、Komadhie C. Dissanayake、Elizabeth J. Gehrmann、Chamari S. Wijesooriya、Atreyee Mukhopadhyay、Emily A. Smith、Arthur H. WinterDOI:10.1021/jacs.0c07139日期:2020.9.9Photocages are light-sensitive chemical protecting groups that give investigators control over activation of biomolecules using targeted light irradiation. A compelling application of far-red/near-IR absorbing photocages is their potential for deep tissue activation of biomolecules and phototherapeutics. Towards this goal, we recently reported BODIPY photocages that absorb near-IR light. However, these光笼是光敏化学保护基团,使研究人员可以使用靶向光照射控制生物分子的激活。远红/近红外吸收光笼的一个引人注目的应用是它们在生物分子和光疗法的深层组织激活方面的潜力。为了实现这一目标,我们最近报道了吸收近红外光的 BODIPY 光笼。然而,与较短波长的吸收光笼相比,这些光笼的光释放效率降低,这阻碍了它们的应用。由于光化学是速率的零和竞争,因此可以通过使所需的光反应更有效或通过阻碍竞争性衰变通道来提高光反应的量子产率。后一种抑制非生产性衰变通道的策略旨在通过合成阻止进入非生产性单线态内部转换锥形交叉点的结构来提高长波长吸收 BODIPY 光笼的释放效率,这些交叉点最近已从激发态动态模拟中定位为简单的 BODIPY 结构. 这种策略导致合成了新的构象限制硼甲基化 BODIPY 光笼,其在 700 nm 附近强烈吸收光。在最好的情况下,光笼的消光系数为 124,000 M-1cm-1,光释放的量子产率为
-
Stepwise iododesilylation and coupling reaction: a flexible route to 2,4-disubstituted-1H-pyrroles作者:Jianhui Liu、Nanyan Fu、Changyun Wei、Zhucao SongDOI:10.1016/j.tetlet.2016.04.105日期:2016.6In this study, we report a new strategy for the synthesis of 2,4-disubstituted pyrroles using a stepwise iododesilylation and palladium-catalysed Suzuki coupling reaction of 2,4-bis(trimethylsilyl)-1-t-Boc-1H-pyrrole (2). The regioselective iodo-desilylation followed by the Suzuki coupling reaction with concomitant Boc deprotection are critical aspects of the new synthesis method.
-
Simple two-step synthesis of 2,4-disubstituted pyrroles and 3,5-disubstituted pyrrole-2-carbonitriles from enones作者:Murat Kucukdisli、Dorota Ferenc、Marcel Heinz、Christine Wiebe、Till OpatzDOI:10.3762/bjoc.10.44日期:——The cyclocondensation of enones with aminoacetonitrile furnishes 3,4-dihydro-2H-pyrrole-2-carbonitriles which can be readily converted to 2,4-disubstituted pyrroles by microwave-induced dehydrocyanation. Alternatively, oxidation of the intermediates produces 3,5-disubstituted pyrrole-2-carbonitriles.
-
Gold(I)-Catalyzed Cascade Hydroarylation/Cycloaromatization to Indolizines via Pyridine Ring Construction作者:Xiangdong Li、Xin Xie、Yuanhong LiuDOI:10.1021/acs.joc.6b00346日期:2016.5.6An efficient and atom-economic method for the synthesis of multisubstituted indolizines via gold-catalyzed cascade hydroarylation/cycloaromatization reaction of α-(N-pyrrolyl)ketones with alkynes is described. The reaction is realized through the construction of the pyridine ring of indolizines, which allows the regioselective incorporation of a wide range of functionalities on the pyridine unit.
表征谱图
-
氢谱1HNMR
-
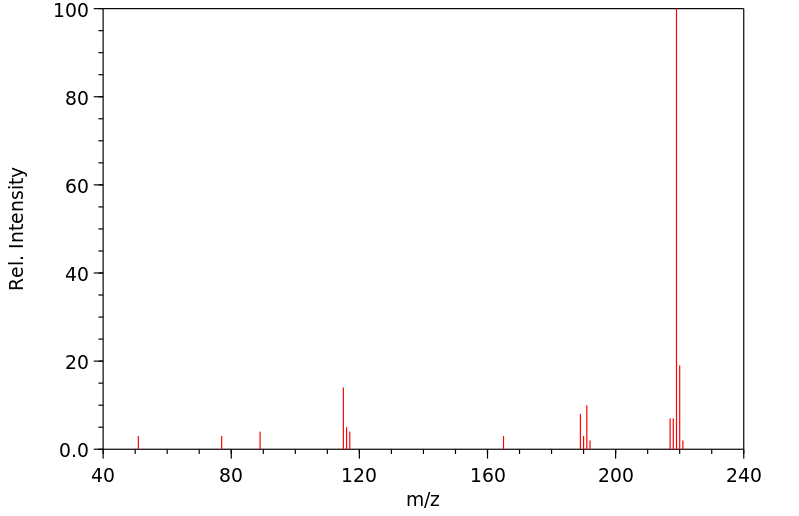
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄胆红酸
高树蛙毒素
颜料红2254
阿根诺卡菌素
阿托伐他汀镁
阿托伐他汀钙阿托伐他汀钙中间体1甲酯
阿托伐他汀钙杂质59
阿托伐他汀钙杂质52
阿托伐他汀钙杂质43
阿托伐他汀钙杂质
阿托伐他汀钙杂质
阿托伐他汀钙三水合物
阿托伐他汀钙L-8
阿托伐他汀钙
阿托伐他汀酸异丙酯
阿托伐他汀酰基-Β-D-葡糖苷酸
阿托伐他汀缩丙酮
阿托伐他汀相关化合物E
阿托伐他汀甲酯
阿托伐他汀甲胺盐
阿托伐他汀烯丙基酯
阿托伐他汀杂质F
阿托伐他汀杂质95
阿托伐他汀杂质5
阿托伐他汀杂质31
阿托伐他汀杂质1
阿托伐他汀叔丁酯
阿托伐他汀双氟杂质中间体
阿托伐他汀内酯-[D5]
阿托伐他汀内酯
阿托伐他汀乙酯
阿托伐他汀USP相关物质E
阿托伐他汀L1二胺物杂质
阿托伐他汀3-羟基消除杂质
阿托伐他汀3-氧杂质
阿托伐他汀
阿利考昔
阿伐他汀钠
镍(II)(吡唑二氰胺)2
镉原卟啉IX二甲酯
铬,二溴二(吡啶)-
达考帕泛
费耐力
角质形成细胞分化诱导剂
西拉美新盐酸盐
西拉美新
虫螨腈
萨格列扎
苏尼替尼N-1
芬度柳