2,6-二溴-4-甲氧基甲苯 | 14542-71-3
中文名称
2,6-二溴-4-甲氧基甲苯
中文别名
3,5-二溴-4-甲基苯甲醚
英文名称
1,3-dibromo-2-methyl-5-methoxybenzene
英文别名
2,6-dibromo-4-methoxytoluene;3,5-dibromo-4-methylanisole;1,3-dibromo-5-methoxy-2-methylbenzene;1,3-Dibrom-5-methoxy-2-methylbenzol;2,6-Dibrom-4-methoxytoluol
CAS
14542-71-3
化学式
C8H8Br2O
mdl
——
分子量
279.959
InChiKey
BZRKRRXRNGVEIZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:271.1±35.0 °C(Predicted)
-
密度:1.727±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2909309090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:储存条件:室温下干燥密封保存。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二溴-4-甲基苯酚 3,5-dibromo-4-methylphenol 13979-81-2 C7H6Br2O 265.932 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3-二溴-2-(溴甲基)-5-甲氧基苯 1,3-dibromo-2-(bromomethyl)-5-methoxybenzene 130445-13-5 C8H7Br3O 358.855 —— 2-(2,6-dibromo-4-methoxyphenyl)acetonitrile 883839-47-2 C9H7Br2NO 304.969 —— N-[(2,6-dibromo-4-methoxyphenyl)methyl]-1-(4-methoxyphenyl)methanamine 444663-76-7 C16H17Br2NO2 415.124 —— 3-(2,6-dibromo-4-methoxyphenyl)-propionic acid 444663-45-0 C10H10Br2O3 337.996 —— t-butyl 3-(2,6-dibromo-4-methoxyphenyl)-propionate 444663-44-9 C14H18Br2O3 394.103 —— 3-Brom-5-methoxy-2-methyl-1-hydroxymethyl-benzol 13979-63-0 C9H11BrO2 231.089 —— 3-Brom-5-methoxy-2-methyl-1-brommethyl-benzol 13979-64-1 C9H10Br2O 293.986
反应信息
-
作为反应物:描述:2,6-二溴-4-甲氧基甲苯 在 盐酸 、 lithium aluminium tetrahydride 、 三溴化磷 、 三氯化铁 、 magnesium 作用下, 生成 3,3'-Dibrom-5,5'-dimethoxy-2,2'-dimethyl-bibenzyl参考文献:名称:Aromatic Molecules Bearing Substituents within the Cavity of the π-Electron Cloud. Synthesis of trans-15,16-Dimethyldihydropyrene摘要:DOI:10.1021/ja00983a028
-
作为产物:描述:参考文献:名称:WO2008/53158摘要:公开号:
文献信息
-
Direct One-Pot Synthesis of Naphthoxindoles from 4-Bromooxindoles by Suzuki-Miyaura Coupling and Aldol Condensation Reactions作者:Kyeong-Yong Park、Bum Tae Kim、Jung-Nyoung HeoDOI:10.1002/ejoc.201301242日期:2014.1An efficient one-pot synthesis of naphthoxindoles by using 4-bromoindolin-2-ones and 2-formylphenylboronic acids has been developed. The coupling reaction proceeds in good to excellent yields under microwave irradiation through a Suzuki–Miyaura coupling and an aldol condensation cascade reaction. In addition, this protocol permits the facile construction of naphthoxindoles through an expanded scope
-
(Halo-benzo carbonyl)heterocyclo fused phenyl p38 kinase inhibiting agents
-
Redox-mediation of electron–electron spin–spin exchange interactions, | J |, in paramagnetic trinuclear molybdenum complexes: an example of a ‘J switch’作者:Elefteria Psillakis、Peter K. A. Shonfield、Abdel-Aziz Jouaiti、John P. Maher、Jon A. McCleverty、Michael D. WardDOI:10.1039/a908323e日期:——A series of trinuclear molybdenum nitrosyl complexes has been prepared using the dinucleating ligands 4-(4-hydroxyphenyl)pyridine (HL1), 1-(4-pyridyl)-2-(4-hydroxyphenyl)-ethene (HL2) and -ethane (HL3), and the trinucleating ligands 3,5-bis(4-hydroxyphenyl)pyridine (H2L4) and 2,6-bis(4-ethenylpyridyl)-4-hydroxytoluene (HL5). These complexes are of the type [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2] (text-decoration:underlineMo = Mo(NO)TpMe,Me, TpMe,Me = tris(3,5-dimethylpyrazolyl)borate; E = nothing, CHCH and CH2CH2; py = C5H4N or C5H3N; from HL1, HL2 and HL3), [Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl] (from H2L4), and [Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}] (from HL5). The species [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2] contains one 16 valence electron (ve) ([text-decoration:underlineMoOC6H4â}2]) and two 17 ve centres ([(âpy)text-decoration:underlineMoCl]), [Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl] has two 16 ([Cltext-decoration:underlineMoOC6H4â}]) and one 17 ([(âpy)text-decoration:underlineMoCl]) ve centres and [Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}] one 16 and two 17 ve centres. Reduction of these species by cobaltocene in tetrahydrofuran/dichloromethane mixtures affords complexes having three 17 ve centres with one unpaired electron per metal centre. The interaction between these unpaired electrons in solution is determined by the relationship between |J|, the electron spinâspin exchange interaction, and AMo, the molybdenum hyperfine coupling constant, which was detected by EPR spectroscopy. In [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2], the interaction was dependent on ligand conformation, |J| â AMo when E = nothing, |J| â«Â AMo when E = CHCH and |J| âªÂ AMo when E = CH2CH2. Reduction of [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2] to [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]â resulted in exchange between all three spins irrespective of ligand conformation, and the EPR spectra of [Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl]2â and [Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}]â were similar to that of [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]â. Oxidation reconstitutes the original EPR spectra of [text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2], [Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl] and [Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}]. This behaviour is consistent with full three centre interaction being âswitched onâ when the 17â¶16â¶17 or 16â¶17â¶16 ve systems are reduced to a 17â¶17â¶17 ve system, and âswitched offâ on reoxidation.一系列三核钼硝酰配合物已经使用二核配体4-(4-羟基苯基)pyridine(HL1)、1-(4-吡啶基)-2-(4-羟基苯基)-乙烯(HL2)和-乙烷(HL3),以及三核配体3,5-二(4-羟基苯基)pyridine(H2L4)和2,6-二(4-乙烯基吡啶)-4-羟基甲苯(HL5)制备。这些配合物的类型为[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2](text-decoration:underlineMo = Mo(NO)TpMe,Me, TpMe,Me = 三(3,5-二甲基吡唑基)硼酸盐;E = nothing, CHCH 和 CH2CH2;py = C5H4N 或 C5H3N;来源于HL1、HL2和HL3),[Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl](来源于H2L4),以及[Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}](来源于HL5)。该物种[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]包含一个16价电子(ve)中心([text-decoration:underlineMoOC6H4–}2])和两个17价电子中心([(-py)text-decoration:underlineMoCl]),而[Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl]具有两个16价电子([Cltext-decoration:underlineMoOC6H4–}])和一个17价电子([(-py)text-decoration:underlineMoCl])中心,以及[Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}]具有一个16和两个17价电子中心。这些物种在四氢呋喃/二氯甲烷混合物中被羧基钴还原会生成具有三个17价电子中心和每个金属中心一个未成对电子的配合物。在溶液中,这些未成对电子之间的相互作用由|J|(电子自旋-自旋交换相互作用)与AMo(钼超细耦合常数)之间的关系决定,这种关系通过EPR光谱法得到检测。在[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]中,相互作用依赖于配体构象,当E = nothing时,|J|≈AMo;当E = CHCH时,|J|≥AMo;当E = CH2CH2时,|J|≤AMo。从[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]还原至[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]⁻时,无论配体构象如何,所有三个自旋之间都发生了交换,而[Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl]⁻和[Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}]⁻的EPR光谱与[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]⁻的光谱相似。氧化过程重新构造了[text-decoration:underlineMoOC6H4Epytext-decoration:underlineMoCl}2]、[Cltext-decoration:underlineMo(OC6H4)}2pytext-decoration:underlineMoCl]和[Cltext-decoration:underlineMoOC6H3Me[CHCHpytext-decoration:underlineMoCl]2}]的原始EPR光谱。这种行为与当17↓16↓17或16↓17↓16价电子体系被还原为17↓17↓17价电子体系时,完全的三中心相互作用被“开启”,而在重新氧化时则被“关闭”的情况相一致。
-
Functionalized Photoreactive Compounds申请人:Cherkaoui Mohammed Zoubair公开号:US20080274304A1公开(公告)日:2008-11-06The present invention concerns functionalized photoreactive compounds of formula (I), that are particularly useful in materials for the alignment of liquid crystals. Due to the adjunction of an electron withdrawing group to specific molecular systems bearing an unsaturation directly attached to two unsaturated ring systems, exceptionally high photosensitivities, excellent alignment properties as well as good mechanical robustness could be achieved in materials comprising said functionalized photoreactive compounds of the invention.本发明涉及式(I)的官能化光反应化合物,特别适用于液晶对准材料。由于在特定分子系统中的不饱和键直接连接到两个不饱和环系统上附加了一个电子受体基团,因此在包含本发明的这些官能化光反应化合物的材料中可以实现异常高的光敏性、优异的对准性能以及良好的机械稳健性。
-
Spin Exchange Interaction through Phenylene-Ethynylene Bridge in Diradicals Based on Iminonitroxide and Nitronylnitroxide Radical Derivatives. 1. Experimental Investigation of the Through-Bond Spin Exchange Coupling作者:Pascale Wautelet、Jacques Le Moigne、Vladimira Videva、Philippe TurekDOI:10.1021/jo034723n日期:2003.10.1pi-conjugation on the intramolecular through-bond spin coupling have been investigated by changing the length of the spacer within linear derivatives. The EPR studies demonstrate the intramolecular magnetic coupling between the radical spins within all compounds. This result is very attractive and unusual, given the large distance between the radicals from 15 A in the dimer to 36 A in the pentamer.
表征谱图
-
氢谱1HNMR
-
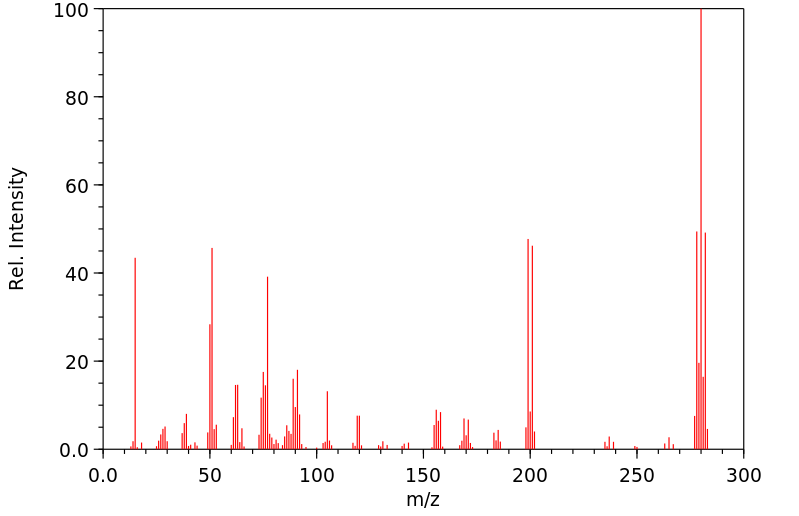
质谱MS
-
碳谱13CNMR
-
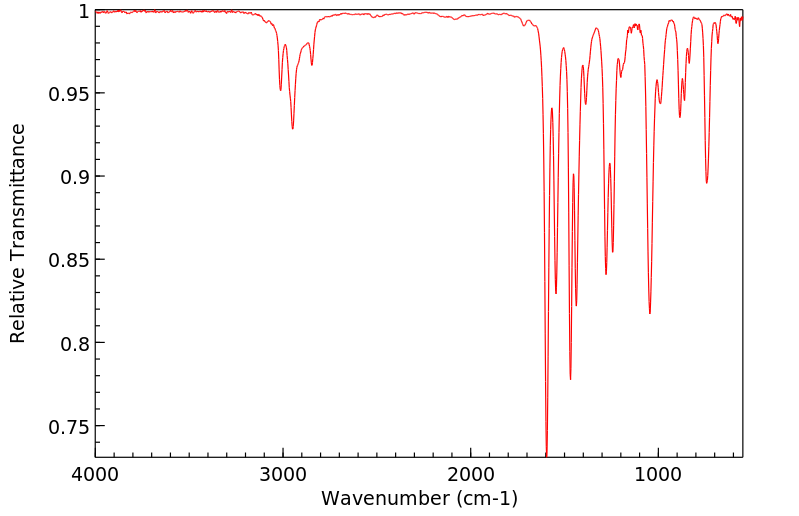
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯