2,6-二甲氧基苯甲腈 | 16932-49-3
中文名称
2,6-二甲氧基苯甲腈
中文别名
2,6-二甲氧基苯腈;2.6-二甲氧基苯腈
英文名称
2,6-dimethoxybenzonitrile
英文别名
2,6-bismethoxybenzonitrile
CAS
16932-49-3
化学式
C9H9NO2
mdl
——
分子量
163.176
InChiKey
XHAHKSSLDJIEDH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:119-123 °C
-
沸点:310 °C
-
密度:1.2021 (rough estimate)
-
稳定性/保质期:
密闭于阴凉干燥环境中。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:42.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S36/37
-
海关编码:2926909090
-
危险品标志:Xn
-
危险类别码:R20/21/22
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存于阴凉干燥处
SDS
| Name: | 2 6-Dimethoxybenzonitrile 97% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 16932-49-3 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 16932-49-3 | 2,6-Dimethoxybenzonitrile | 97 | 241-000-2 |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin. May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Most nitrile compounds are well absorbed through intact skin, and may cause delayed onset of symptoms following exposure by this route.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration. Inhalation may result in symptoms similar to cyanide poisoning which include tachypnea, hyperpnea (abnormally rapid or deep breathing), and dyspnea (labored breathing) followed rapidly by respiratory depression. Pulmonary edema may occur.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Get medical aid immediately. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Approach fire from upwind to avoid hazardous vapors and toxic decomposition products. Combustion by-products include oxides of nitrogen and hydrogen cyanide.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 16932-49-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: light beige
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 310 deg C @ 760 mm Hg
Freezing/Melting Point: 119 - 123 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H9NO2
Molecular Weight: 163.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, cyanides.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 16932-49-3: DI4355400 LD50/LC50:
Not available.
Carcinogenicity:
2,6-Dimethoxybenzonitrile - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 16932-49-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 16932-49-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 16932-49-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (2,6-二甲氧基苯基)甲醇 2,6-dimethoxybenzyl alcohol 16700-55-3 C9H12O3 168.192 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-hydroxy-6-methoxybenzonitrile 71590-96-0 C8H7NO2 149.149 2,6-二羟基苯甲腈 2,6-dihydroxybenzonitrile 57764-46-2 C7H5NO2 135.122 2,6-二甲氧基苄胺 2,6-Dimethoxybenzylamine 20781-22-0 C9H13NO2 167.208 —— 3-bromo-2,6-dimethoxy-benzonitrile 872857-96-0 C9H8BrNO2 242.072
反应信息
-
作为反应物:描述:2,6-二甲氧基苯甲腈 在 kieselguhr-catalyst 、 nickel-copper 作用下, 生成 间苯二甲醚参考文献:名称:Catalytic Hydrogenation of Aromatic Nitrites摘要:DOI:10.1246/bcsj.32.861
-
作为产物:描述:参考文献:名称:磁性可回收的氢氧化镧作为伯醇有氧氧化转化为腈的有效催化剂摘要:在这里,我们报告了一种新颖的可磁性回收的氢氧化镧纳米颗粒,用于直接从相应的醇中以氨为氮源氧化合成腈。研究了La(OH)3 / Fe 3 O 4磁性纳米粒子的制备和表征方法,并探讨了该方法的范围和一般性,适用于一系列具有给电子和吸电子基团的结构多样的伯醇。当在O 2气氛下在回流条件下使用相对于苄醇为5摩尔%的La时,观察到最佳结果。La(OH)3 / Fe 3 O 4 可以使用外部磁体轻松地将磁性纳米颗粒从反应混合物中分离出来,并重复使用至少5次,而不会显着降低活性。DOI:10.1002/aoc.4253
文献信息
-
Transformation of aromatic bromides into aromatic nitriles with n-BuLi, pivalonitrile, and iodine under metal cyanide-free conditions作者:Ko Uchida、Hideo TogoDOI:10.1016/j.tet.2019.130550日期:2019.9by the treatment of aryl bromides with n-butyllithium and then pivalonitrile, followed by the treatment with molecular iodine at 70 °C, without metal cyanides under transition-metal-free conditions. The present reaction proceeds through the radical β-elimination of imino-nitrogen-centered radicals formed from the reactions of imines and N-iodoimines under warming conditions. © 2019 Elsevier Science
-
A Metal‐Free Direct Arene C−H Amination作者:Tao Wang、Marvin Hoffmann、Andreas Dreuw、Edina Hasagić、Chao Hu、Philipp M. Stein、Sina Witzel、Hongwei Shi、Yangyang Yang、Matthias Rudolph、Fabian Stuck、Frank Rominger、Marion Kerscher、Peter Comba、A. Stephen K. HashmiDOI:10.1002/adsc.202100236日期:2021.6.8The synthesis of aryl amines via the formation of a C−N bond is an essential tool for the preparation of functional materials, active pharmaceutical ingredients and bioactive products. Usually, this chemical connection is only possible by transition metal-catalyzed reactions, photochemistry or electrochemistry. Here, we report a metal-free arene C−H amination using hydroxylamine derivatives under benign
-
Catalytic oxidative conversion of alcohols, aldehydes and amines into nitriles using KI/I2–TBHP system作者:K. Rajender Reddy、C. Uma Maheswari、M. Venkateshwar、S. Prashanthi、M. Lakshmi KantamDOI:10.1016/j.tetlet.2009.02.057日期:2009.5The oxidative conversion of alcohols, aldehydes and amines to give corresponding nitriles in excellent yields was easily achieved by the catalytic amount of KI or I2 in combination with TBHP as an external oxidant. This non-transition metal catalyst is cost effective and provides easy work-up and separation of the product.
-
Tandem Retro-Michael Addition-Claisen Rearrangement-Intramolecular Cyclization: One-Pot Synthesis of Densely Functionalized Ethyl Dihydropyrimidine-4-carboxylates from Simple Building Blocks作者:B. NaiduDOI:10.1055/s-2008-1032088日期:——Pyrolysis of suitably functionalized oxadiazoline diesters in refluxing xylenes provided ethyl 1,2-disubstituted 5-hydroxy-6-oxo-1,6-dihydropyrimidine-4-carboxylates in moderate to good yields. Under thermal conditions, the oxadiazoline esters undergo a sequence of complex molecular reorganizations, namely retro-Michael addition, Claisen rearrangement, and cyclization, to produce the desired pyrimidinones.
-
Cu<sub>2</sub>O-Mediated Room Temperature Cyanation of Aryl Boronic Acids/Esters and TMSCN作者:Yong Ye、Yanhua Wang、Pengtang Liu、Fushe HanDOI:10.1002/cjoc.201200711日期:2013.1A method for the efficient and reliable synthesis of aryl nitriles via the Cu2O‐catalyed cross‐coupling of aryl boronic acids or esters and TMSCN is presented. A broad range of substrates decorated by electron‐rich and deficient, sterically very congested, and labile functionalities were tolerated. Moreover, the reaction can proceed under mild conditions at room temperature. These advantages paired
表征谱图
-
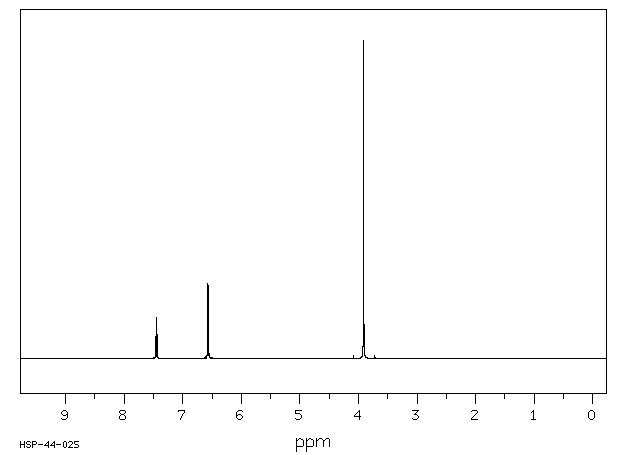
氢谱1HNMR
-
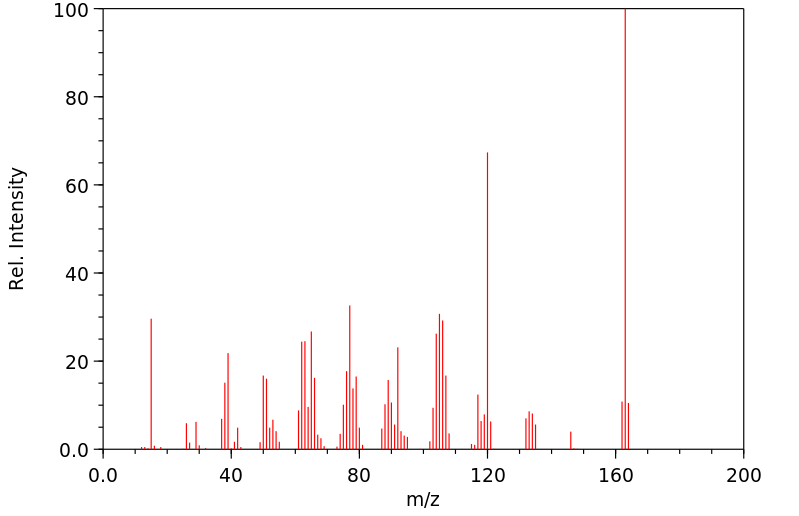
质谱MS
-
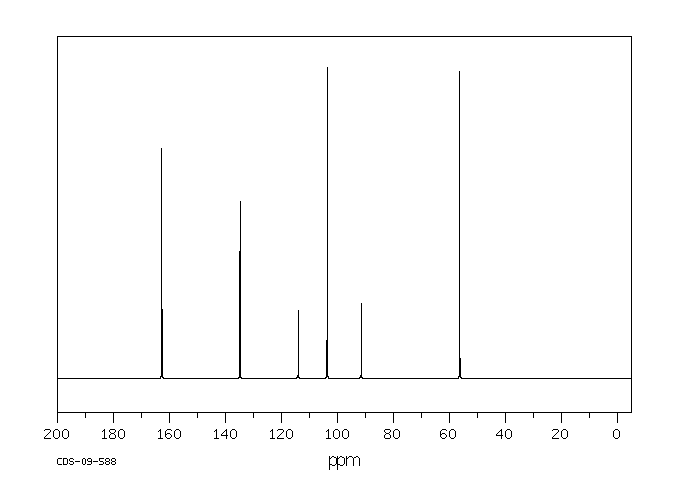
碳谱13CNMR
-
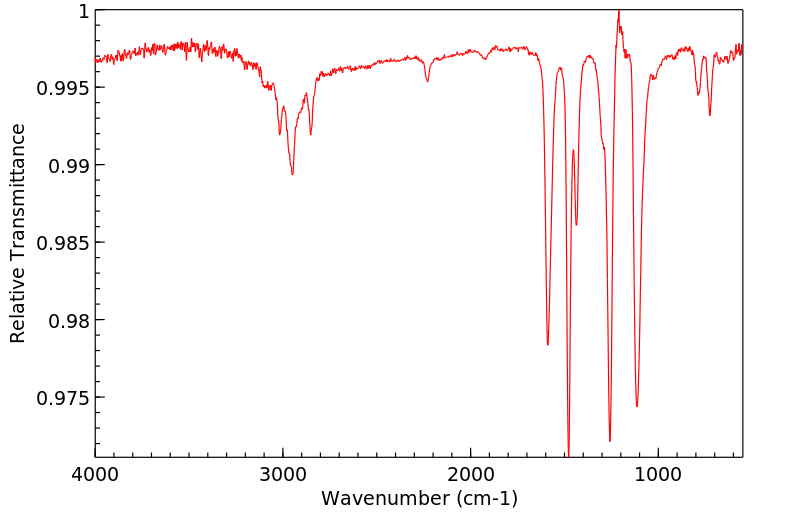
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫