diethyl (azulene)-1,3-dicarboxylate | 36044-39-0
中文名称
——
中文别名
——
英文名称
diethyl (azulene)-1,3-dicarboxylate
英文别名
Diethyl azulene-1,3-dicarboxylate;1,3-diethoxycarbonylazulene;azulene-1,3-dicarboxylic acid diethyl ester;Azulen-1,3-dicarbonsaeure-diaethylester;Azulen-1.3-dicarbonsaeurediaethylester;1.3-Azulendicarbonsaeurediaethylester;1,3-Diethoxycarbon ylazulene
CAS
36044-39-0
化学式
C16H16O4
mdl
——
分子量
272.301
InChiKey
RVUQIDQFWHDMPK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:120-121 °C
-
沸点:399.5±15.0 °C(Predicted)
-
密度:1.171±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:20
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— diethyl 6-bromoazulene-1,3-dicarboxylate 1157-45-5 C16H15BrO4 351.197 二乙基2-氯-1,3-薁二羧酸酯 diethyl 2-chloroazulene-1,3-dicarboxylate 36044-40-3 C16H15ClO4 306.746 —— diethyl 6-(tri-n-butylstannyl)azulene-1,3-dicarboxylate 441292-60-0 C28H42O4Sn 561.349 2-氨基甘菊环-1,3-二甲酸二乙酯 diethyl 2-aminoazulene-1,3-dicarboxylate 3806-02-8 C16H17NO4 287.315 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3-Azulendicarbonsaeure-dimethylester 51381-43-2 C14H12O4 244.247 1,3-薁二羧酸,6-羟基-,二乙基酯 diethyl 6-hydroxy-1,3-azulenedicarboxylate 1157-46-6 C16H16O5 288.3 —— Diethyl 4-methylazulene-1,3-dicarboxylate 50883-04-0 C17H18O4 286.328 二乙基6-氨基薁-1,3-二羧酸酯 6-aminoazulene-1,3-dicarboxylic acid diethyl ester 13148-46-4 C16H17NO4 287.315 —— Diethyl 6-tert-butylazulene-1,3-dicarboxylate 50883-08-4 C20H24O4 328.408 —— diethyl 6-allyloxy-1,3-azulenedicarboxylate 260409-16-3 C19H20O5 328.365 —— Diethyl 6-naphthalen-1-ylazulene-1,3-dicarboxylate 50883-07-3 C26H22O4 398.458 —— azulene-1,3-dicarboxylic acid 38303-39-8 C12H8O4 216.193 —— Diethyl 2-phenylazulene-1,3-dicarboxylate 50883-01-7 C22H20O4 348.398 —— Diethyl 2-methylazulene-1,3-dicarboxylate 51031-11-9 C17H18O4 286.328 —— Diethyl 2,5-diphenylazulene-1,3-dicarboxylate 52457-23-5 C28H24O4 424.496 —— Diethyl 2,6-diphenylazulene-1,3-dicarboxylate 52457-22-4 C28H24O4 424.496 —— ethyl azulene-1-carboxylate 1206-88-8 C13H12O2 200.237 —— Diethyl 4-tert-butylazulene-1,3-dicarboxylate 50883-06-2 C20H24O4 328.408 —— Diethyl 2-naphthalen-1-ylazulene-1,3-dicarboxylate 50883-03-9 C26H22O4 398.458 —— Diethyl 4-phenylazulene-1,3-dicarboxylate 50883-02-8 C22H20O4 348.398 —— Diethyl 4-(α-naphthyl)azulene-1,3-dicarboxylate 50883-05-1 C26H22O4 398.458 —— Diethyl 2,4-diphenylazulene-1,3-dicarboxylate 52457-20-2 C28H24O4 424.496 —— Diethyl 2-methyl-4-phenylazulene-1,3-dicarboxylate 313063-40-0 C23H22O4 362.425 - 1
- 2
反应信息
-
作为反应物:描述:diethyl (azulene)-1,3-dicarboxylate 在 叔丁基过氧化氢 、 potassium tert-butylate 作用下, 以 四氢呋喃 、 正己烷 、 氨 为溶剂, 反应 4.0h, 以83%的产率得到1,3-薁二羧酸,6-羟基-,二乙基酯参考文献:名称:通过氢的替代亲核取代对芘烯进行羟基化和胺化摘要:当 5 元环中含有吸电子取代基时,菘烯与叔丁基过氧化氢在 6 位有效地进行羟基化。类似地,通过 4-氨基-1,2,4-三唑对芘进行 VNS 胺化;它的阴离子是一种活性亲核试剂,也与未取代的薁烯反应。报道了 6-羟基甘菊烯的各种转化,例如用氮、氧、硫、碳亲核试剂和卤素取代相应的磺酸盐,以及烯丙基醚的克莱森重排。DOI:10.1002/(sici)1099-0690(200001)2000:1<193::aid-ejoc193>3.0.co;2-w
-
作为产物:描述:2-甲氧基-2,4,6-环庚三烯-1-酮 在 硫酸 、 sodium ethanolate 、 亚硝酸异戊酯 作用下, 以 乙醇 为溶剂, 生成 diethyl (azulene)-1,3-dicarboxylate参考文献:名称:The Synthesis of Azulene Derivatives from Troponoids摘要:DOI:10.1246/bcsj.35.1179
文献信息
-
Preparation and Stille cross-coupling reaction of the first organotin reagents of azulenes. Easy access to poly(azulen-6-yl)benzene derivatives作者:Shunji Ito、Tetsuo Okujima、Noboru MoritaDOI:10.1039/b203836f日期:2002.8.8afforded 1,4-di-, 1,3,5-tri-, 1,2,4,5-tetra-, 1,2,4-tri- and 1,2,3,5-tetra(azulen-6-yl)benzene derivatives (18, 20, 22, 24 and 25). The redox behavior of 18 and 22 was examined by cyclic voltammetry (CV) and compared with those of 20 and 24 reported previously. In contrast to the three-step reduction of 20, the compound 18 exhibited a reversible one-step two-electron reduction wave at −1.30 V upon CV,第一种通用的有机金属试剂衍生自 天青石,即,6-(三- Ñ -butylstannyl)薁(1a)及其1,3-二乙氧羰基衍生物(1b),是由Pd(0)催化直接制备的。锡烷基化的与双6- bromoazulenes(三- Ñ -butyltin)。我们展示了该试剂在Stille交叉偶联反应中的实用性芳基, 酰基和a烯基卤化物以良好的收率得到6-芳基-,6-酰基-和双-azulenes。此外,该方法被应用于合成聚(azulen-6-基)苯衍生物。1b与1,4-二-,1,3,5-三-,1,2,4,5-四-和六溴苯的反应得到1,4-二-,1,3,5-三-, 1,2,4,5-四- ,1,2,4-三-和1,2,3,5-四(薁-6-基)苯衍生物(18,20,22,24和25)。18和22的氧化还原行为通过循环伏安法 (简历),并与之前报告的20和24进行比较。与20的三步还原相反,化合物18表现出可逆的一步两电子减少
-
Reactions of diethyl azulene-1,3-dicarboxylate derivatives and 1-azaazulene derivatives with Grignard reagents, and alkyl- and aryllithium作者:Tadayoshi Morita、Noritaka Abe、Kahei TakaseDOI:10.1039/b003078n日期:——Reactions of diethyl azulene-1,3-dicarboxylate (1) and diethyl 2-chloroazulene-1,3-dicarboxylate (11) with Grignard reagents, followed by dehydration with tetrachloro-1,2-benzoquinone, gave 2-, 4,- and 6-substituted addition–oxidation products. Grignards reagents have a tendency to react with 1 at the positions in the order of 2 > 4 > 6, while steric hindrance has a greater influence at the positions in order of 2 > 4 > 6. Reactions of 1 and 11 with phenyllithium and methyllithium gave similar results. On the other hand, on the reaction of diethyl 2-methoxyazulene-1,3-dicarboxylate (15), Grignard reagents attacked preferentially at the methoxy group, and 2-substituted products were obtained. Use of excess molar equivalents of Grignard reagents led to diaryl-substituted products. Reaction of 2-chloro-1-azaazulenes with Grignard reagents also gave similar addition–oxidation products, and reacted at the positions in the order 8 ≫ 4 > 6, whereas reaction of 2-methoxy-1-azaazulene with a Grignard reagent gave 1-azaazulen-2(1H)-one.二乙基azulene-1,3-二羧酸酯(1)和二乙基2-氯azulene-1,3-二羧酸酯(11)与格氏试剂反应,随后与四氯-1,2-苯并醌脱水,生成了2-、4-和6-取代的加成-氧化产物。格氏试剂倾向于按照2 > 4 > 6的顺序与1反应,而立体位阻在2 > 4 > 6的位置上有更大的影响。1和11与苯基锂和甲基锂的反应结果相似。另一方面,二乙基2-甲氧基azulene-1,3-二羧酸酯(15)的反应中,格氏试剂优先攻击甲氧基,得到2-取代产物。使用过量的格氏试剂会导致二芳基取代产物的形成。2-氯-1-氮杂azulenes与格氏试剂的反应也生成类似的加成-氧化产物,反应位置的顺序为8 ≫ 4 > 6,而2-甲氧基-1-氮杂azulene与格氏试剂的反应则生成1-氮杂azulen-2(1H)-酮。
-
Synthesis of 1,1′-, 2,2′-, 1,2′-, and 2,6′-Biazulenes and Their Derivatives by Ullmann Reaction作者:Tadayoshi Morita、Kahei TakaseDOI:10.1246/bcsj.55.1144日期:1982.4Four kinds of biazulenes, 1,1′- (1a), 2,2′- (2a), 1,2′- (3a), and 2,6′-biazulenes (4a), and their derivatives were synthesized by utilizing Ullmann-type coupling of haloazulene derivatives. Ullmann reaction of ethyl 3-iodo-(5a) and 2-iodoazulene-1-carboxylates (6b) gave diethyl 1,1′-biazulene-3,3′-dicarboxylate (1b) and diethyl 2,2′-biazulene-1,1′-dicarboxylate (2b), respectively, in excellent yields1,1'- (1a), 2,2'- (2a), 1,2'- (3a), 2,6'-双脒(4a) 4种及其衍生物合成卤代芘衍生物的乌尔曼型偶联。3-碘-(5a) 和 2-iodoazulene-1-carboxylates (6b) 的 Ullmann 反应得到 1,1'-biazulene-3,3'-dicarboxylate (1b) 和 2,2'-biazulene-1 二乙酯,1'-二羧酸盐 (2b),分别以极好的收率。2-碘芴反应直接得到2a。二乙基 2-chloroazulene-1,3-dicarboxylate (7a) 和它的 5-烷基衍生物也反应得到四乙基 2,2'-biazulene-1,1',3,3'-tetracarboxylate (2d) 和它的 5,分别为 5'-二烷基衍生物。5a 和 6b 的混合 Ullmann 反应提供了 1b、2b 和 1,2'-联茚-1'
-
Preparation and Stille cross-coupling reaction of the first organotin reagents of azulenes. An efficient Pd(0)-catalyzed synthesis of 6-aryl- and biazulenes作者:Tetsuo Okujima、Shunji Ito、Noboru MoritaDOI:10.1016/s0040-4039(01)02347-4日期:2002.2The first versatile organometallic reagents of azulenes, 6-(tri-n-butylstannyl)azulenes, have been prepared by a Pd(0)-catalyzed direct stannylation of 6-bromoazulenes with bis(tri-n-butyltin). We demonstrate herein the utility of the reagents in the Stille cross-coupling reaction with aryl and azulenyl halides to afford 6-aryl- and biazulenes in good yield.
-
A transition metal catalysed photoreaction. Ferrocene-sensitized photoalkoxycarbonylation of azulene
表征谱图
-
氢谱1HNMR
-
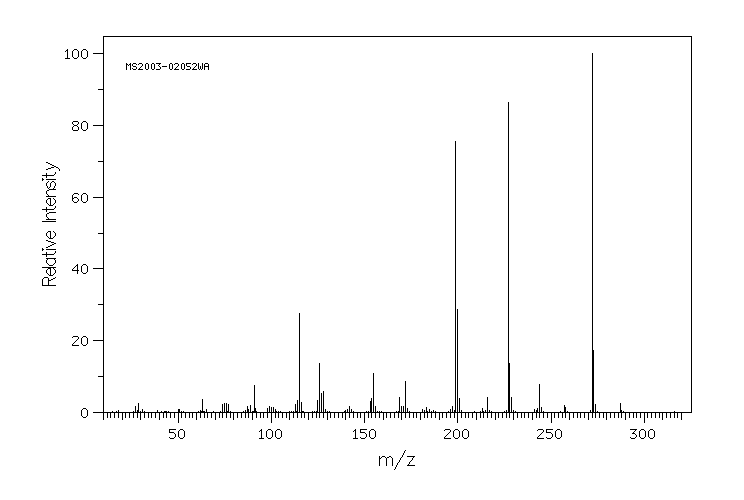
质谱MS
-
碳谱13CNMR
-
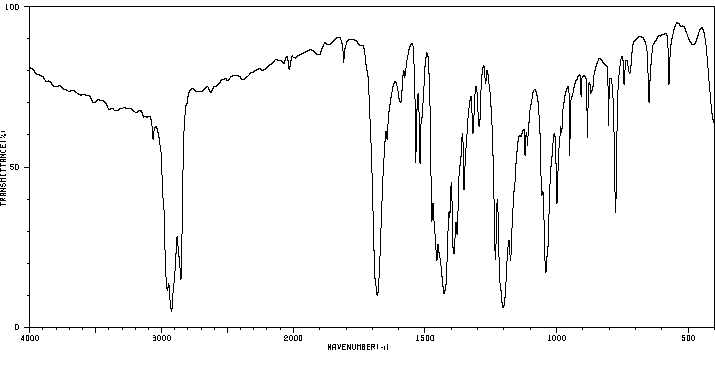
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-