烯亚丙基二乙酸酯 | 869-29-4
物质功能分类
中文名称
烯亚丙基二乙酸酯
中文别名
烯丙亚基二乙酸酯
英文名称
1,1-diacetoxy-2-propene
英文别名
prop-2-ene-1,1-diyl diacetate;allylidene diacetate;3,3-diacetoxypropene;1-acetyloxyprop-2-enyl acetate
CAS
869-29-4
化学式
C7H10O4
mdl
MFCD00008708
分子量
158.154
InChiKey
TXECTBGVEUDNSL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-37.6°C
-
沸点:180°C (estimate)
-
密度:1.0760
-
溶解度:0.06 M
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:11
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.428
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1(a)
-
危险类别码:R23/24/25,R34,R36/37/38
-
危险品运输编号:UN 2810
-
海关编码:2915390090
-
包装等级:II
-
安全说明:S26,S36/37/39
SDS
制备方法与用途
反应信息
-
作为反应物:参考文献:名称:硝酸铈铵催化醛类化学选择性合成酰基醛及其脱保护的温和高效方法摘要:已经开发了一种温和有效的方法,用于在催化量的硝酸铈铵存在下使用乙酸酐从醛化学选择性合成双乙酸酯(酰基),收率极好。发现酮在反应条件下不受影响。还实现了用水和硝酸铈铵对酰基进行脱保护。DOI:10.1055/s-2002-34243
-
作为产物:参考文献:名称:通过分支选择性 Pd/Cu 催化的烯丙基取代对简单二羧酸盐进行立体发散去对称化。摘要:不对称去对称化已被证明是在不对称合成中构建立体中心的有力策略。本文报道了 Pd/Cu 催化的不对称去对称化反应与简单的偕二羧酸盐。带有芳基或杂芳基的多种亚氨基酯与这种双金属催化体系相容。反应顺利进行,以良好的收率得到所需的产物,具有高至优异的区域选择性、非对映选择性和对映选择性(高达 20:1 的支链:线性,>20:1 dr,>99 % ee)。值得注意的是,该反应有利于支链选择性,这对于 Pd 催化的烯丙基烷基化反应是不寻常的。此外,标准产品可以很容易地转化为其他有价值的分子,例如手性烯丙醇、氨基甲酸酯和有机硼化合物。DOI:10.1002/chem.202200273
-
作为试剂:参考文献:名称:Process for the preparation of 8-hydroxyquinoline摘要:通过将邻硝基苯酚溶液逐渐加入丙烯醛或烯丙基醚或二酯的水酸溶液中的邻氨基酚,可以获得纯级8-羟基喹啉的产量显著增加。8-羟基喹啉是一种商业产品,具有已知用途。公开号:US04044011A1
文献信息
-
A NOVEL AND EFFICIENT CONVERSION OF ALDEHYDES TO 1,1-DIACETATES CATALYZED WITH FeCl<sub>3</sub>/SiO<sub>2</sub>UNDER MICROWAVE IRRADIATION作者:Cunde Wang、Minghua LiDOI:10.1081/scc-120014779日期:2002.1ABSTRACT 1,1-Diacetates (3) are effectively synthesized in few minutes by acylation reaction of aldehydes with acetic anhydride in the presence of FeCl3/SiO2 under microwave irradiation.
-
An Improved Method for the Synthesis of Allylic<i>gem</i>-Diacetates from α,β-Unsaturated Aldehydes Catalyzed by Lithium Tetrafluoroborate作者:Fumiaki Ono、Kuniaki Nishioka、Shirou Itami、Hirotaka Takenaka、Tsuneo SatoDOI:10.1246/cl.2008.1248日期:2008.12.5An improved method for the synthesis of allylic gem-diacetates (acylals) is described. The desired acylals are obtained by the reaction of α,β-unsaturated aldehydes with acetic anhydride using a catalytic amount of lithium tetrafluoroborate in diethyl ether at room temperature.
-
RuCl<sub>3</sub> · <i>x</i>H<sub>2</sub>O: A New Efficient Catalyst for Facile Preparation of 1,1‐Diacetates from Aldehydes作者:Anil Saini、Sanjay Kumar、Jagir. S. SandhuDOI:10.1080/00397910701650831日期:2007.12.1Abstract An efficient, facile preparation of aldehyde 1,1‐diacetates (acylals) in excellent yields catalyzed by RuCl3 · xH2O is described. Ketones do not react under these conditions.摘要 描述了在 RuCl3·xH2O 催化下以优异的产率高效、简便地制备醛 1,1-二乙酸酯(酰基)。酮在这些条件下不反应。
-
Zinc Tetrafluoroborate-Catalyzed Efficient Conversion of Aldehydes to Geminal Diacetates and Cyanoacetates作者:Brindaban C. Ranu、Jyotirmoy Dutta、Arijit DasDOI:10.1246/cl.2003.366日期:2003.4A trace of an aqueous solution of zinc tetrafluoroborate was demonstrated to catalyze the conversion of an aldehyde to its 1,1-diacetate by acetic anhydride without any solvent. A similar reaction of an aldehyde with a mixture of potassium cyanide and acetic anhydride in methylene chloride was also catalyzed by Zn(BF4)2 to provide the corresponding geminal cyanoacetate.
-
A Remarkable HBF<sub>4</sub>-SiO<sub>2</sub>-Catalyzed Synthesis of Acylals from Aldehydes under Solvent-Free Conditions作者:Vinod Kamble、Babasaheb Bandgar、Neeta Joshi、Vasant JamodeDOI:10.1055/s-2006-950282日期:2006.10HBF 4 -SiO 2 has been found to be an outstanding catalyst for the protection of carbonyl compounds as acylals under entirely solvent-free conditions. Some of the major advantages of this procedure are high yields, ease of operation, high chemoselectivity, high atom efficiency, and compatibility with other protecting groups.已发现 HBF 4 -SiO 2 是一种出色的催化剂,用于在完全无溶剂的条件下保护作为酰基的羰基化合物。该方法的一些主要优点是收率高、易于操作、化学选择性高、原子效率高以及与其他保护基团的相容性。
表征谱图
-
氢谱1HNMR
-
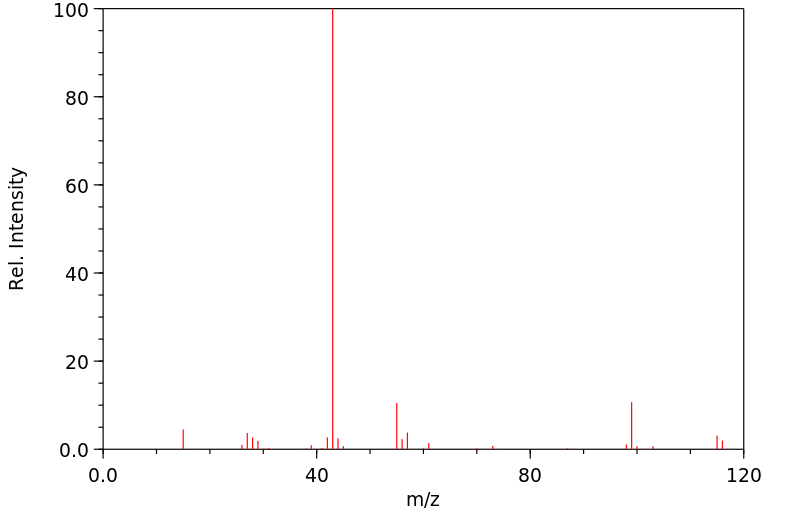
质谱MS
-
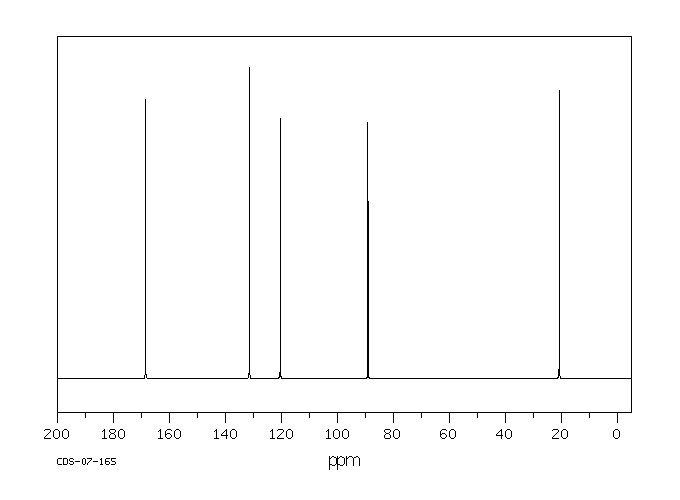
碳谱13CNMR
-
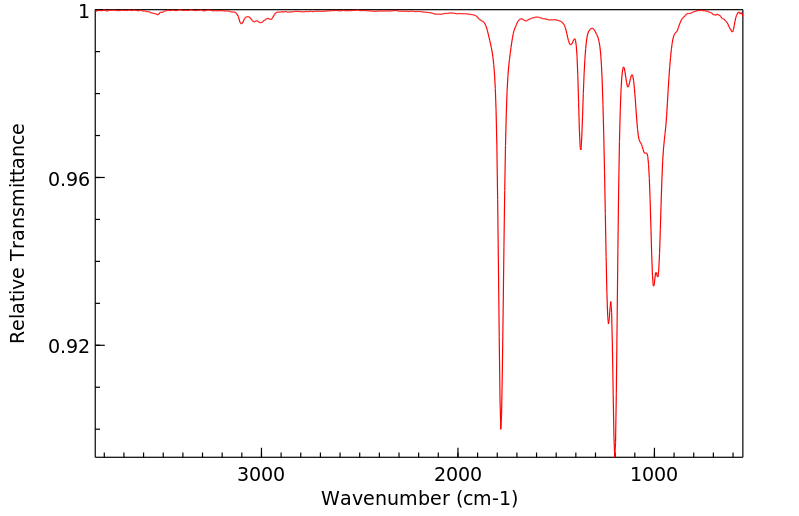
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸