2-溴-6-硝基甲苯 | 55289-35-5
中文名称
2-溴-6-硝基甲苯
中文别名
1-溴-2-甲基-3-硝基苯;2-甲基-3-硝基溴苯
英文名称
2-bromo-6-nitrotoluene
英文别名
1-bromo-2-methyl-3-nitrobenzene;6-bromo-2-nitrotoluene
CAS
55289-35-5
化学式
C7H6BrNO2
mdl
MFCD00009792
分子量
216.034
InChiKey
LYTNSGFSAXWBCA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:38-40 °C (lit.)
-
沸点:143 °C/22 mmHg (lit.)
-
密度:1.6841 (rough estimate)
-
闪点:>110°C
-
保留指数:1345.8
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,并且没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2904909090
-
危险品运输编号:UN 2810 6.1/PG 1
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器保持密封状态,并存放在阴凉、干燥的地方。确保工作环境有良好的通风或排气设施。
SDS
2-溴-6-硝基甲苯 修改号码:5
模块 1. 化学品
产品名称: 2-Bromo-6-nitrotoluene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
2-溴-6-硝基甲苯 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-溴-6-硝基甲苯
百分比: >98.0%(GC)
CAS编码: 55289-35-5
分子式: C7H6BrNO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
2-溴-6-硝基甲苯 修改号码:5
模块 7. 操作处置与储存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体
颜色: 极淡的黄色-黄色
气味: 无资料
pH: 无数据资料
熔点:
40°C
沸点/沸程 136 °C/1.1kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
2-溴-6-硝基甲苯 修改号码:5
模块 12. 生态学信息
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Bromo-6-nitrotoluene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
2-溴-6-硝基甲苯 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-溴-6-硝基甲苯
百分比: >98.0%(GC)
CAS编码: 55289-35-5
分子式: C7H6BrNO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
2-溴-6-硝基甲苯 修改号码:5
模块 7. 操作处置与储存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体
颜色: 极淡的黄色-黄色
气味: 无资料
pH: 无数据资料
熔点:
40°C
沸点/沸程 136 °C/1.1kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
2-溴-6-硝基甲苯 修改号码:5
模块 12. 生态学信息
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
应用 2-溴-6-硝基甲苯可作为医药合成的中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基硝基苯 1-methyl-2-nitrobenzene 88-72-2 C7H7NO2 137.138 3-溴-2-甲基苯胺 3-bromo-2-methylaniline 55289-36-6 C7H8BrN 186.051 2-甲基-3-硝基苯胺 3-nitro-o-tolylamine 603-83-8 C7H8N2O2 152.153 2,6-二硝基甲苯 2,6-Dnt 606-20-2 C7H6N2O4 182.136 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-6-硝基苄溴 1-bromo-2-(bromomethyl)-3-nitrobenzene 58579-54-7 C7H5Br2NO2 294.93 2-溴-6-硝基苯甲醛 2-bromo-6-nitrobenzaldehyde 20357-21-5 C7H4BrNO3 230.018 —— 1-bromo-2-ethenyl-3-nitrobenzene 90481-68-8 C8H6BrNO2 228.045 2-溴-6-硝基苯甲醇 (2-bromo-6-nitrophenyl)methanol 861106-91-4 C7H6BrNO3 232.034 —— 2-bromo-6-nitrophenylacetylene 916978-57-9 C8H4BrNO2 226.029 2-溴-6-硝基苯乙醇 2-(2-bromo-6-nitrophenyl)ethan-1-ol 118665-02-4 C8H8BrNO3 246.06 —— [(E)-2-(2-Bromo-6-nitro-phenyl)-vinyl]-dimethyl-amine 105205-47-8 C10H11BrN2O2 271.114 —— 2-(2-bromo-6-nitrophenyl)ethoxycarbonyl chloride 179691-25-9 C9H7BrClNO4 308.516 甲基硝基苯 1-methyl-2-nitrobenzene 88-72-2 C7H7NO2 137.138 2-(2-溴-6-硝基苯基)乙酸 6-bromo-2-nitrophenylacetic acid 37777-74-5 C8H6BrNO4 260.044 1-溴-2-甲基-3,4-二硝基苯 1-bromo-2-methyl-3,4-dinitrobenzene 290353-57-0 C7H5BrN2O4 261.032 —— 2-(2-bromo-6-nitrophenyl)ethyl methanesulfonate 301645-39-6 C9H10BrNO5S 324.152 2-溴-6-硝基苯甲酸 2-bromo-6-nitrobenzoic acid 38876-67-4 C7H4BrNO4 246.017 —— o-Nitro-6-brom-phenyl-propiolsaeure-methylester 21213-98-9 C10H6BrNO4 284.066 (2-溴-6-硝基苄氧基)(叔丁基)二甲基硅烷 ((2-bromo-6-nitrobenzyl)oxy)(tert-butyl)dimethylsilane 1147531-02-9 C13H20BrNO3Si 346.296 3-溴-2-甲基苯胺 3-bromo-2-methylaniline 55289-36-6 C7H8BrN 186.051 —— 2-bromo-6-nitrophenylacetylene diethylphosphonate 916978-58-0 C12H13BrNO5P 362.117 —— 2,2,2-Trichloroethyl 3-(2-bromo-6-nitrophenyl)prop-2-ynoate 947612-45-5 C11H5BrCl3NO4 401.428 2-溴-6-硝基苯甲酸甲酯 methyl 2-bromo-6-nitrobenzoate 135484-76-3 C8H6BrNO4 260.044 6-溴-2-硝基苯基丙酮酸 6-bromo-2-nitrophenylpyruvic acid 98592-11-1 C9H6BrNO5 288.054 —— 3-(2-Bromo-6-nitrophenyl)-1-(4-chlorophenyl)prop-2-yn-1-one 947612-48-8 C15H7BrClNO3 364.582 —— methyl 3-(2'-bromo-6'-nitrophenyl)-2-oxopropanoate 862718-99-8 C10H8BrNO5 302.081 - 1
- 2
- 3
反应信息
-
作为反应物:描述:2-溴-6-硝基甲苯 在 盐酸 、 四(三苯基膦)钯 、 铁粉 、 cesium fluoride 作用下, 以 乙二醇二甲醚 、 乙醇 为溶剂, 反应 6.0h, 生成 3-(3-Methoxyphenyl)-2-methylaniline参考文献:名称:Discovery of a series of (4,5-Dihydroimidazol-2-yl)-biphenylamine 5-HT7 agonists摘要:A novel (4,5-dihydroimidazol-2-yl)-biphenylamine series of 5-HT7 agonist compounds was developed from a structurally related lead compound 1. The newly discovered series is exemplified by compound 2 that possesses high affinity for 5-HT7 receptors and shows intrinsic agonist activity in functional assays. This new series has significant alpha(1) and alpha(2) activities perhaps due to the presence of the 2-aminoimidazoline moiety. (C) 2002 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(02)00925-3
-
作为产物:描述:参考文献:名称:作为 FtsZ 抑制剂的新型 4-溴-1H-吲唑衍生物的合成和抗菌活性摘要:设计、合成了一系列作为丝状温度敏感蛋白 Z (FtsZ) 抑制剂的新型 4-溴-1H-吲唑衍生物,并测定了它们对各种表型革兰氏阳性菌和革兰氏阴性菌的体外抗菌活性。细胞分裂抑制活性。结果表明,该系列对表皮葡萄球菌和对青霉素敏感的化脓性链球菌的抗菌活性优于其他受试菌株。其中,化合物12和18对耐青霉素金黄色葡萄球菌的活性是3-甲氧基苯甲酰胺(3-MBA)的256倍和256倍,化合物18的活性是3-MBA的64倍,但4-在抑制金黄色葡萄球菌 ATCC29213 方面的活性比环丙沙星弱一倍。特别,化合物 9 对化脓性链球菌 PS 表现出最佳活性 (4 µg/mL),分别比 3-MBA、姜黄素和环丙沙星的活性高 32 倍、32 倍和 2 倍,但为 4 倍活性低于苯唑西林钠。此外,一些合成的化合物对金黄色葡萄球菌 ATCC25923、大肠杆菌 ATCC25922 和铜绿假单胞菌 ATCC27853 的DOI:10.1002/ardp.201400412
文献信息
-
Heterocyclic sulfonamide derivatives申请人:——公开号:US20030225127A1公开(公告)日:2003-12-04The present invention provides certain heterocyclic sulfonamide derivatives of formula (I): useful for potentiating glutamate receptor function in a patient and therefore, useful for treating a wide variety of conditions, such as psychiatric and neurological disorders.
-
INDOLINONE ANALOGUES申请人:ENGELHARDT Harald公开号:US20140296229A1公开(公告)日:2014-10-02The present invention encompasses compounds of general formula (I) wherein the groups R 1 to R 4 , A 1 and A 2 have the meanings given in the claims and in the specification. The compounds of the invention are suitable for the treatment of diseases characterized by excessive or abnormal cell proliferation pharmaceutical preparations containing such compounds and their uses as a medicament.本发明涵盖了一般式(I)的化合物, 其中基团R1至R4,A1和A2具有权利要求和说明书中给定的含义。本发明的化合物适用于治疗以细胞过度或异常增殖为特征的疾病,包括含有这些化合物的药物制剂以及它们作为药物的用途。
-
Synthesis of 123I-Labelled Analogues of Imidazobenzodiazepine Receptor Ligands.作者:Meredith E. McPhee、Andrew G. Katsifis、Filomena Mattner、Damon D. RidleyDOI:10.1071/ch99135日期:——
Reaction of bromo- or iodo-substituted isatoic anhydrides with N-methylglycine, L-proline or D-proline afforded bromo- or iodo-substituted 1,4-benzodiazepinediones which on condensation with ethyl or t-butyl isocyanoacetates gave ethyl or t-butyl bromo- or iodo-imidazobenzodiazepine carboxylates. These aryl halides were converted into the corresponding tributylstannanes with bis(tributyltin) in the presence of (triphenylphosphine)palladium(0), and the stannanes were treated with sodium (123I)iodide in the presence of chloramine-Tto give the required 123I- labelled analogues of the imidazobenzodiazepine receptor ligands flumazenil and bretazenil.
溴代或碘代异丁烯酸酐与 N-甲基甘氨酸、L-脯氨酸或 D-脯氨酸反应,得到溴代或碘代的 1,4-苯并二氮杂环庚二酮。 或碘代的 1,4-苯并二氮杂环庚二酮,在与乙基或叔丁基异氰基乙酸酯缩合后 异氰基乙酸乙酯或异氰基乙酸叔丁酯缩合后,得到溴代或碘代咪唑苯并 碘咪唑并二氮杂卓羧酸酯。这些芳基卤化物 在(三苯基膦)存在下,用双(三丁基锡)将这些芳基卤化物转化为相应的三丁基锡烷。 三苯基膦)钯(0)的存在下,用双(三丁基锡)将这些芳基卤化物转化为相应的三丁基锡烷,然后用 在氯胺-T 的存在下,用(123I)碘化钠处理锡烷,得到所需的 123I 标记。 得到所需的 123I 标记的类似物 咪唑苯并二氮杂卓受体配体氟马西尼和溴他西尼。 -
Pd-<sup> <i>t</i> </sup> BuONO Cocatalyzed Aerobic Indole Synthesis作者:Xiao-Shan Ning、Xin Liang、Kang-Fei Hu、Chuan-Zhi Yao、Jian-Ping Qu、Yan-Biao KangDOI:10.1002/adsc.201701512日期:2018.4.17A Pd‐tBuONO co‐catalyzed scalable and practical synthesis of indoles with molecular oxygen as terminal oxidant is developed. Either terminal or internal 2‐vinylanilines could be smoothly converted to desired indoles under one general condition. This method has been evaluated in the large scale synthesis of indomethacin and a potential anti‐breast cancer drug candidate 1.
-
Steroid receptor modulator compounds and methods申请人:Ligand Pharmaceuticals Incorporated公开号:US05696127A1公开(公告)日:1997-12-09Non-steroidal compounds which are high affinity, high selectivity modulators for steroid receptors are disclosed. Also disclosed are pharmaceutical compositions incorporating such compounds, methods for employing the disclosed compounds and compositions for treating patients requiring steroid receptor agonist or antagonist therapy, intermediates useful in the preparation of the compounds and processes for the preparation of the steroid receptor modulator compounds.
表征谱图
-
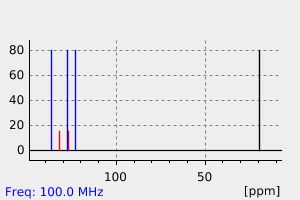
氢谱1HNMR
-
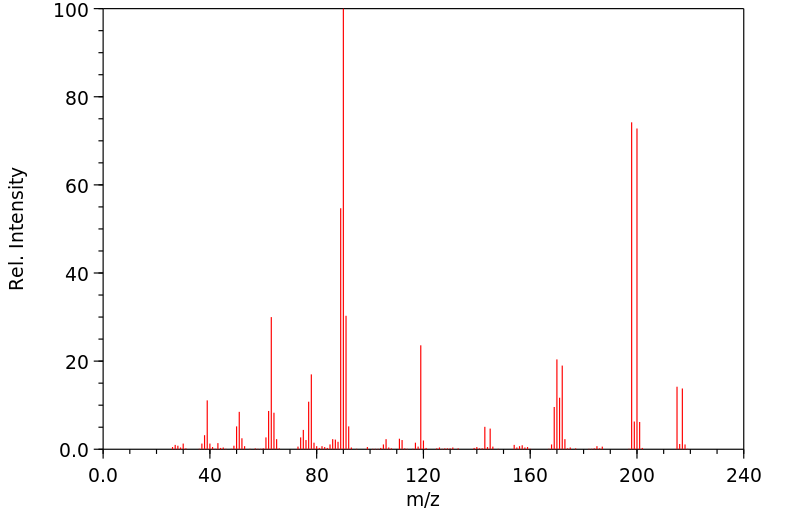
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫