2-甲基-5-硝基苯并咪唑 | 1792-40-1
中文名称
2-甲基-5-硝基苯并咪唑
中文别名
2-甲基-5-硝基-1H-苯并咪唑;2-甲基-6-硝基苯并咪唑;1H-苯并咪唑,2-甲基-5-硝基-;苯并咪唑,2-甲基-5-硝基-;2-甲基-6-硝基-1H-苯并咪唑
英文名称
2-methyl-5-nitrobenzimidazole
英文别名
2-methyl-5-nitro-1H-benzimidazole;2-methyl-5-nitro-1H-benzo[d]imidazole;2-methyl-6-nitro-benzimidazole;5(6)-nitro 2-methyl benzimidazole
CAS
1792-40-1
化学式
C8H7N3O2
mdl
MFCD00005599
分子量
177.162
InChiKey
RKRXTVLCZDPERO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:223-225 °C
-
沸点:446.0±18.0 °C(Predicted)
-
密度:1.437±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:74.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥环境中使用。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Methyl-5-nitro-1H-1,3-benzodiazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Methyl-5-nitro-1H-1,3-benzodiazole
CAS number: 1792-40-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7N3O2
Molecular weight: 177.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Methyl-5-nitro-1H-1,3-benzodiazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Methyl-5-nitro-1H-1,3-benzodiazole
CAS number: 1792-40-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7N3O2
Molecular weight: 177.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-methyl-5,6-dinitro-1H-benzoimidazole 89939-69-5 C8H6N4O4 222.16 1,2-二甲基-5-硝基-1H-苯并咪唑 1,2-dimethyl-5-nitro-1H-benzimidazole 49819-79-6 C9H9N3O2 191.189 5-氨基-2-甲基苯并咪唑 5-amino-2-methylbenzimidazole 29043-48-9 C8H9N3 147.18 1,2-二甲基-6-硝基-1H-苯并咪唑 1,2-dimethyl-6-nitro-1H-benzoimidazole 19492-66-1 C9H9N3O2 191.189 —— 1-ethyl-2-methyl-5-nitro-1H-benzoimidazole 21163-13-3 C10H11N3O2 205.216 —— 2-methyl-4,6(5,7)-dinitrobenzimidazole 92303-12-3 C8H6N4O4 222.16 1-乙基-2-甲基-6-硝基-1H-苯并咪唑 1-ethyl-2-methyl-6-nitro-1H-benzimidazole 103273-08-1 C10H11N3O2 205.216 —— 2-(2-methyl-5-nitro-1H-benzimidazol-1-yl)acetonitrile 913706-12-4 C10H8N4O2 216.199 —— 1-allyl-2-methyl-5-nitro-1H-benzo[d]imidazole 1434623-20-7 C11H11N3O2 217.227 —— 2-(5-nitro-1H-benzo[d]imidazol-1-yl)ethanol 84382-34-3 C9H9N3O3 207.189 —— 1-allyl-2-methyl-6-nitro-1H-benzimidazole 1434623-12-7 C11H11N3O2 217.227 (9CI)-(2-甲基-1H-苯并咪唑-5-基)-脲 1-(2-Methyl-1H-benzo[d]imidazol-5-yl)urea 72550-37-9 C9H10N4O 190.2 N-(2-甲基-1H-苯并[d]咪唑-5-基)-乙酰胺 5-acetamido-2-methylbenzimidazole 56842-62-7 C10H11N3O 189.217 1-苄基-2-甲基-5-硝基-1H-1,3-苯并咪唑 1-Benzyl-2-methyl-5-nitro-1H-benzimidazole 14624-88-5 C15H13N3O2 267.287 1,2-二甲基-1H-苯并咪唑-5-胺双盐酸盐 1,2-dimethyl-1H-benzimidazol-5-amine 3527-19-3 C9H11N3 161.206 —— 1-hydrazinyl-2-(2-methyl-5-nitro-1H-benzimidazol-1-yl)acetonitrile 913706-13-5 C10H12N6O2 248.244 —— Pentanoic acid (2-methyl-1H-benzoimidazol-5-yl)-amide 82326-37-2 C13H17N3O 231.297 —— 2-methyl-N-(pyridin-2-ylmethyl)-3H-benzimidazol-5-amine 1157762-95-2 C14H14N4 238.292 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:具有抗癌活性的5-取代2-甲基苯并咪唑的合成摘要:3-(2-甲基-nz-1H-be 5)的硫代羧酰亚胺基吡唑基5、苯基吡唑基6、二甲基吡唑基7、硝基苯基吡唑基8、二甲基恶唑基9、苯并恶氮杂10和嘧啶基11a-c衍生物的一系列化合物ylazo) 戊烷-2, 4-二酮被合成。此外,5-氨基-2-甲基苯并咪唑(3)与邻苯二甲酸酐或马来酸酐在乙酸或甲苯中反应生成12-15。用氰基乙酸乙酯或丙二酸二乙酯或乙酰丙酮处理 5, 6-二氨基-2-甲基苯并咪唑 (16) 导致形成苯二氮卓衍生物 17-20。针对 60 种人类癌细胞系测试了化合物 2、7、9、10 和 11 的细胞毒活性。发现化合物 7 和 9 是最有效的。DOI:10.1002/ardp.200390005
-
作为产物:描述:参考文献:名称:[EN] 3-AZABICYCLO [3.1.0] HEXANE DERIVATIVES AS VANILLOID RECEPTOR LIGANDS
[FR] DÉRIVÉS DE 3-AZABICYCLO[3.1.0]HEXANE UTILISÉS COMME LIGANDS DES RÉCEPTEURS VANILLOÏDES摘要:公开号:WO2009090548A3
文献信息
-
Simple and chemoselective reduction of aromatic nitro compounds to aromatic amines: reduction with hydriodic acid revisited作者:J.S.Dileep Kumar、ManKit M Ho、Tatsushi ToyokuniDOI:10.1016/s0040-4039(01)01083-8日期:2001.8Reduction of aromatic nitro compounds to amines with hydriodic acid was reinvestigated. Under a milder non-refluxing condition (at 90°C for 2–4 h), the reduction proceeded efficiently with excellent chemoselectivity without affecting other functional groups including nitrile, ester, halide, carbonyl, amide, sulfonamide, imidazole and methylthio groups.
-
Rhodium catalyzed 2‐alkyl‐benzimidazoles synthesis from benzene‐1,2‐diamines and tertiary alkylamines as alkylating agents作者:Yamini、Saurabh Sharma、Pralay DasDOI:10.1002/aoc.6278日期:2021.8synthesized from benzene-1,2-diamine and tertiary amines as alkylating agent under polystyrene supported rhodium (Rh@PS) nanoparticles (NPs) catalyzed conditions. The heterogeneous rhodium catalyst was applied first time for the synthesis of 2-alkyl-benzimidazoles. The reaction followed through oxidation of alkylamines, transamination, and oxidative cyclisation with benzene-1,2-diamines for the corresponding
-
Imidazolium chloride-catalyzed synthesis of benzimidazoles and 2-substituted benzimidazoles from o-phenylenediamines and DMF derivatives作者:Zongjie Gan、Qingqiang Tian、Suqin Shang、Wen Luo、Zeshu Dai、Huajun Wang、Dan Li、Xuetong Wang、Jianyong YuanDOI:10.1016/j.tet.2018.11.014日期:2018.12general, and economical synthesis of diversely functionalized benzimidazoles and 2-substituted benzimidazoles has been realized via the imidazolium chloride-catalyzed cyclization of o-phenylenediamines with DMF derivatives. This protocol shows a broad substrate scope for aliphatic, aromatic, and heteroaromatic amides. A series of benzimidazoles and 2-substituted benzimidazoles have been obtained in moderate
-
Nanoporous TiO<sub>2</sub> containing an ionic liquid bridge as an efficient and reusable catalyst for the synthesis of <i>N</i>,<i>N</i>′-diarylformamidines, benzoxazoles, benzothiazoles and benzimidazoles作者:M. Mazloumi、F. Shirini、O. Goli-Jolodar、M. SeddighiDOI:10.1039/c8nj00171e日期:——In this work, a green and efficient procedure is reported for the preparation of N,N'-diarylformamidines, benzoxazoles, benzothiazoles, and benzimidazoles using nanoporous TiO2 containing an ionic liquid bridge. This reagent is prepared via the modification of nanoporous TiO2 with bis-3-(trimethoxysilylpropyl)-ammonium hydrogen sulfate (TiO2-[bip]-NH2+ HSO4−). The procedure gave the products in excellent
-
Expeditious and Efficient Synthesis of Benzoxazoles, Benzothiazoles, Benzimidazoles Catalyzed by Ga(OTf)3 under Solvent-Free Conditions作者:Juyan Liu、Qian Liu、Wei Xu、Weilu WangDOI:10.1002/cjoc.201180310日期:2011.8new and efficient method for the synthesis of benzoxazoles, benzothiazoles, benzimidazoles from reactions of o‐substituted aminoaromatics with orthoesters in the presence of catalytic amounts of Ga(OTf)3 under solvent‐free conditions is presented. The remarkable features of this new protocol are high conversion, very short reaction times, cleaner reaction profiles under solvent‐free conditions, straight
表征谱图
-
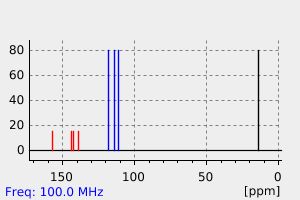
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10