3,4-二甲基氯苄 | 102-46-5
中文名称
3,4-二甲基氯苄
中文别名
3,4-二甲基苄基氯
英文名称
4-(chloromethyl)-1,2-dimethylbenzene
英文别名
3,4-dimethylbenzyl chloride;1-chloromethyl-3,4-dimethylbenzene;1,2-dimethyl-4-(chloromethyl)benzene
CAS
102-46-5
化学式
C9H11Cl
mdl
MFCD00000910
分子量
154.639
InChiKey
UBQRAAXAHIKWRI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-43.35°C (estimate)
-
沸点:116-117 °C24 mm Hg(lit.)
-
密度:1.056 g/mL at 25 °C(lit.)
-
闪点:205 °F
-
保留指数:1233;1200
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:8
-
危险品标志:C
-
危险类别码:R34,R36/37
-
危险品运输编号:UN 3265 8/PG 2
-
WGK Germany:3
-
海关编码:2903999090
-
包装等级:III
-
危险类别:8
-
安全说明:S26,S36/37/39,S45
-
储存条件:2-8°C
SDS
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 1. 化学品
产品名称: 3,4-Dimethylbenzyl Chloride (coNTains isomer)
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,4-二甲基氯苄(含异构体)
百分比: >70.0%(GC)
CAS编码: 102-46-5
俗名: 1-(Chloromethyl)-3,4-dimethylbenzene (coNTains isomer)
分子式: C9H11Cl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 8. 接触控制和个体防护
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 117 °C/3.2kPa
闪点: 96°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.06
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强碱
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 12. 生态学信息
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 3265
正式运输名称: 腐蚀性液体, 酸性的, 有机的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3,4-Dimethylbenzyl Chloride (coNTains isomer)
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,4-二甲基氯苄(含异构体)
百分比: >70.0%(GC)
CAS编码: 102-46-5
俗名: 1-(Chloromethyl)-3,4-dimethylbenzene (coNTains isomer)
分子式: C9H11Cl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 8. 接触控制和个体防护
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 117 °C/3.2kPa
闪点: 96°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.06
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强碱
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
3,4-二甲基氯苄(含异构体) 修改号码:6
模块 12. 生态学信息
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 3265
正式运输名称: 腐蚀性液体, 酸性的, 有机的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,4-三甲基苯 1,2,4-Trimethylbenzene 95-63-6 C9H12 120.194 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2,4-tris-chloromethyl-benzene 2735-07-1 C9H9Cl3 223.529 3,4-二甲基苯甲醛 3,4-dimethylbenzaldehyde 5973-71-7 C9H10O 134.178
反应信息
-
作为反应物:描述:参考文献:名称:Kulka, Canadian Journal of Research, Section B: Chemical Sciences, 1945, vol. 23, p. 106,107摘要:DOI:
-
作为产物:参考文献:名称:Aromatische Spirane, 20. Mitt.: Darstellung von dimethylsubstituierten 2-Carboxymethyl-indan-1-onen und Benzylchloriden als Synthone f�r Synthesen von di- bis tetramethylierten 2,2?-Spirobiindandionen摘要:The isomeric dimethyl methylbenzoates 5, obtained from the bromides via Grignard reactions with dimethylcarbonate, were reduced with LiAlH4 to the hydroxymethyl derivatives 6. The latter were then transformed both to the benzylchlorides 7 (with SOCl2) and to the aldehydes 8 (with pyridinium chlorochromate). Knoevenagel-Doebner reaction of 8 afforded the acrylic acids 9 which (after hydrogenation to 11) were cyclized to the desired indanones 12 with polyphosphoric acid. On the other hand, 12c and 12e were prepared from dimethyl 3-chloropropiophenone (14) by warming with sulfuric acid. After NaH-catalyzed reaction with dimethylcarbonate, the indanones 12 gave the ketoesters 15 which then could be hydrogenated to the indanes 16. All reactions proceeded with satisfactory to excellent yields (60-90%).DOI:10.1007/bf00807400
文献信息
-
Oxidation of Benzyl Halides to Aldehydes and Ketones with Potassium Nitrate Catalyzed by Phase-Transfer Catalyst in Aqueous Media作者:Qifa Liu、Ming Lu、Feng Sun、Jiang Li、Yuebing ZhaoDOI:10.1080/00397910802323080日期:2008.11.3Abstract The catalytic oxidation of benzyl halides to aldehydes and ketones in aqueous media was studied under relatively mild reaction conditions by using phase-transfer catalyst combined with potassium nitrate and 10% aqueous potassium hydroxide solution. As a result, a simple high-yield procedure has been developed.
-
Simple, High Yield Preparation of<i>N</i>-Benzylacetamides by Lewis Acid-Catalyseed Reaction of Benzyl Chlorides or Benzyl Methyl Ethers with Acetonitrile作者:Mesut Kacan、Alexander McKillopDOI:10.1080/00397919308018613日期:1993.8Abstract Reaction of either benzyl chlorides or benzyl methyl ethers with hydrated ferric chloride in acetonitrile results in smooth Ritter reaction and formation of N-benzylacetamides in excellent yield.
-
Synthesis and antirhinovirus activity of 6-(dimethylamino)-2-(trifluoromethyl)-9-(substituted benzyl)-9H-purines作者:James L. Kelley、James A. Linn、J. W. T. SelwayDOI:10.1021/jm00128a016日期:1989.8A series of 6-(dimethylamino)-2-(trifluoromethyl)-9-(substituted benzyl)purines was synthesized and tested for antirhinovirus activity. Most of the compounds were synthesized by alkylation of 6-chloro-2-(trifluoromethyl)-9H-purine with the appropriate benzyl halide followed by displacement of the chloro group with dimethylamine. Alternatively, 6-(dimethylamino)-2-(trifluoromethyl)purine was alkylated
-
N6-Substituted Adenosine Receptor Agonists. Synthesis and Pharmacological Activity as Potent Antinociceptive Agents作者:Timur Gungor、Patrice Malabre、Jean-Marie Teulon、Francoise Camborde、Joelle Meignen、Francoise Hertz、Angela Virone-Oddos、Francois Caussade、Alix CloarecDOI:10.1021/jm00051a007日期:1994.12N6-(indol-3-yl)alkyl derivatives of adenosine were synthesized. The adenosine receptor affinity and the antinociceptive activity of these compounds were assessed in binding studies and the phenylbenzoquinone-induced writhing test. Most of these analogues exhibited a potent analgesic activity without side effects. Among them, compound 3c (UP 202-32) bound to A1 (Ki = 110 nM) and A2 (Ki = 350 nM) adenosine receptors
-
Substituted phenyl farnesyltransferase inhibitors申请人:——公开号:US20020019527A1公开(公告)日:2002-02-14Compounds of formula (I) 1 or pharmaceutically acceptable salts thereof, inhibit farnesyltransferase. Methods for making the compounds, pharmaceutical compositions containing the compounds, and methods of treatment using the compounds are disclosed.式(I)的化合物或其药学上可接受的盐,抑制法尼基转移酶。公开了制备这些化合物的方法,含有这些化合物的药物组合物,以及使用这些化合物进行治疗的方法。
表征谱图
-
氢谱1HNMR
-
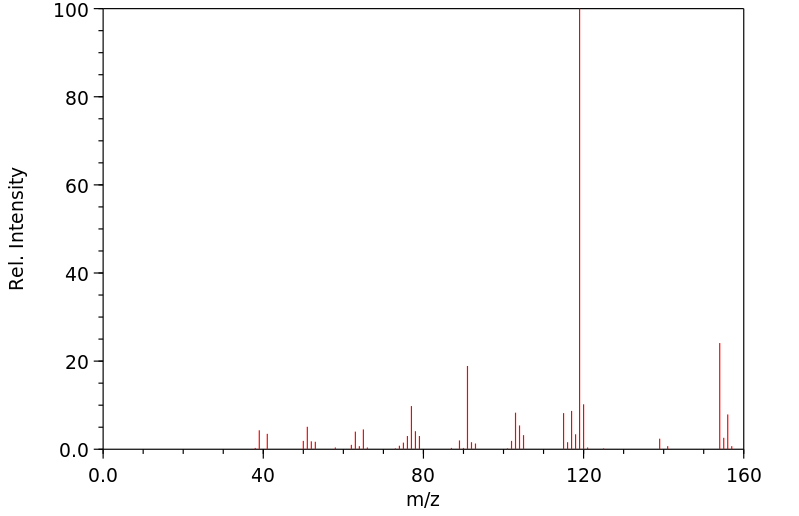
质谱MS
-
碳谱13CNMR
-
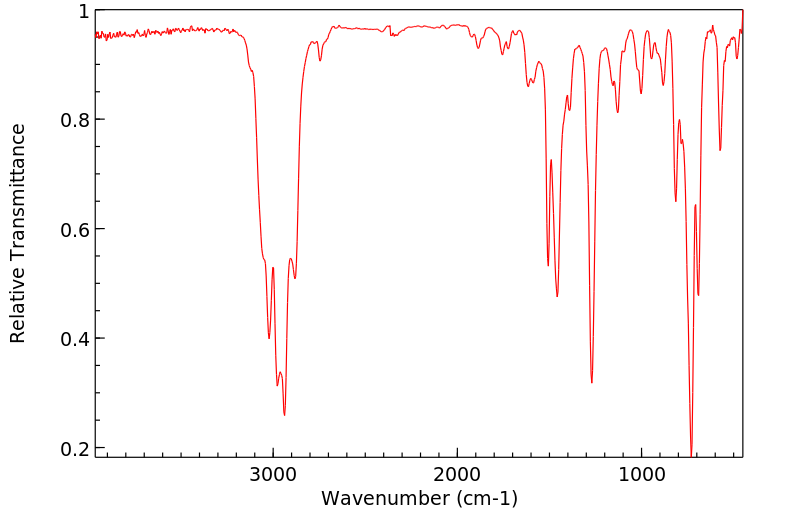
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫