dihydroeudesmol | 6770-16-7
中文名称
——
中文别名
——
英文名称
dihydroeudesmol
英文别名
β-eudesmol;(4aR)-1c.4ar-dimethyl-7c-(α-hydroxy-isopropyl)-(8atH)-decalin;Dihydro-β-eudesmol;Dihydroendesmol;2-[(2R,4aR,8S,8aS)-4a,8-dimethyl-2,3,4,5,6,7,8,8a-octahydro-1H-naphthalen-2-yl]propan-2-ol
CAS
6770-16-7
化学式
C15H28O
mdl
——
分子量
224.387
InChiKey
YJHVMPKSUPGGPZ-SFDCQRBFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:82 °C
-
沸点:155-160 °C(Press: 12.5 Torr)
-
密度:0.933±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 beta-桉叶醇 (±)-β-eudesmol 473-15-4 C15H26O 222.371
反应信息
-
作为反应物:描述:参考文献:名称:萜类化合物—LXXXI:elemol和eudesmol的转化产物以及elemanones和selinanones的合成摘要:用痕量的高氯酸在乙酸中处理后,四氢大麦醇和二氢大麦醇进行脱水。当将所得的烃进行(i)环氧化,然后用BF 3-醚酸酯处理,(ii)硼氢化-氧化,然后用Jones铬酸试剂进一步氧化时,分别得到电子体酮和硒代壬酮。(-)elemane-8-one(VIII)和(-)selinane-8-one(XXIII)可从反应混合物中通过半咔唑类化合物以纯净状态分离。(±)Elemane-8-一可通过焦磷酸马格隆加氢而获得。不能以纯净状态获得受阻酮elemane-6-one(VII)和selinane-6-one(XXII)或它们相应的醇。讨论了各种产品的立体化学,并研究了其ORD曲线。DOI:10.1016/0040-4020(66)80155-2
-
作为产物:描述:参考文献:名称:萜类化合物—LXXXI:elemol和eudesmol的转化产物以及elemanones和selinanones的合成摘要:用痕量的高氯酸在乙酸中处理后,四氢大麦醇和二氢大麦醇进行脱水。当将所得的烃进行(i)环氧化,然后用BF 3-醚酸酯处理,(ii)硼氢化-氧化,然后用Jones铬酸试剂进一步氧化时,分别得到电子体酮和硒代壬酮。(-)elemane-8-one(VIII)和(-)selinane-8-one(XXIII)可从反应混合物中通过半咔唑类化合物以纯净状态分离。(±)Elemane-8-一可通过焦磷酸马格隆加氢而获得。不能以纯净状态获得受阻酮elemane-6-one(VII)和selinane-6-one(XXII)或它们相应的醇。讨论了各种产品的立体化学,并研究了其ORD曲线。DOI:10.1016/0040-4020(66)80155-2
文献信息
-
Structures and Spasmolytic Activities of Derivatives from Sesquiterpenes of Alpinia speciosa and Alpinia japonica.作者:Makoto MORITA、Hiroshi NAKANISHI、Hiroshi MORITA、Susumu MIHASHI、Hideji ITOKAWADOI:10.1248/cpb.44.1603日期:——Sesquiterpenes isolated from Alpinia speciosa and Alpinia japonica, and their derivatives were found to inhibit histamine- or barium chloride-induced contraction of excised guinea pig ileum when tested by the Magnus method. Major spasmolytic principles contained in those extracts were the sesquiterpenes, β-eudesmol, nerolidol, humulene epoxide II and 4α-hydroxydihydroagarofuran. Relationships between the chemical structures of the sesquiterpenes and their derivatives, and their spasmolytic activities were discussed.
-
Terpenoids—LXI作者:K.R. Varma、S.C. BhattacharyyaDOI:10.1016/s0040-4020(01)98514-x日期:1964.1Dihydro-β-eudesmol (IV), obtained by catalytic hydrogenation of β-eudesmol (II), on pyrolysis via the benzoate gives dihydro-β-selinene (V). Its epoxyderivative (VI) is converted on treatment with acetic acid to the hydroxy acetate (VII), the benzoate of which on pyrolysis, followed by saponification, affords dihydrocostol (XI), converted by hydrogenation to tetrahydrocostol (XII). The alcohol (XI)
-
Polyterpene und Polyterpenoide LIX. Über die Synthese von für die Stereochemie der Sesquiterpene wichtigen alkylierten trans-Dekalinen作者:L. Ruzicka、D. R. Koolhaas、Alida H. WindDOI:10.1002/hlca.19310140523日期:1931.10.1
-
Korthals,H.-P. et al., Justus Liebigs Annalen der Chemie, 1971, vol. 745, p. 39 - 58作者:Korthals,H.-P. et al.DOI:——日期:——
-
Zur Kenntnis der Sesquiterpene. (56. Mitteilung). Über den Abbau des Dihydro-eudesmols mit Chromsäure作者:L. Ruzicka、Pl. A. Plattner、A. FürstDOI:10.1002/hlca.19420250625日期:1942.10.15
表征谱图
-
氢谱1HNMR
-
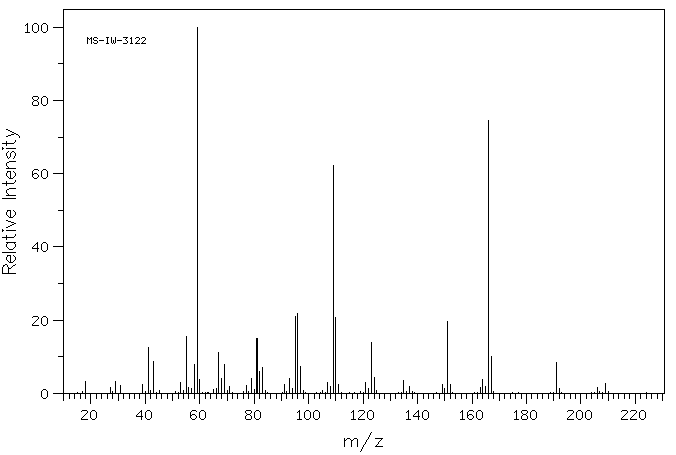
质谱MS
-
碳谱13CNMR
-
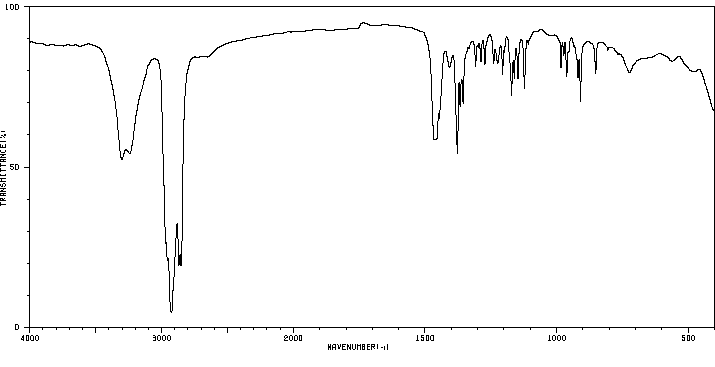
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸