3-氯丙酸苯酯 | 24552-27-0
中文名称
3-氯丙酸苯酯
中文别名
——
英文名称
phenyl 3-chloropropanoate
英文别名
phenyl 3-chloropropionate;Phenyl-(β-chlor-propionat)
CAS
24552-27-0
化学式
C9H9ClO2
mdl
MFCD00045298
分子量
184.622
InChiKey
RAFRTSDUWORDLA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:157 °C / 30mmHg
-
密度:1,2 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S36,S37,S39
-
危险类别码:R36/37/38
-
海关编码:2915900090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
SDS
Phenyl 3-Chloropropionate Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Phenyl 3-Chloropropionate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Phenyl 3-Chloropropionate
Percent: >95.0%(GC)
CAS Number: 24552-27-0
Synonyms: 3-Chloropropionic Acid Phenyl Ester
Chemical Formula: C9H9ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Phenyl 3-Chloropropionate
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: clear
Phenyl 3-Chloropropionate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Color: Colorless - Pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 157 °C/4kPa
Flash Point: No data available
Explosive limits
No data available
Lower:
Upper: No data available
1.20
Density:
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Phenyl 3-Chloropropionate
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Phenyl 3-Chloropropionate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Phenyl 3-Chloropropionate
Percent: >95.0%(GC)
CAS Number: 24552-27-0
Synonyms: 3-Chloropropionic Acid Phenyl Ester
Chemical Formula: C9H9ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Phenyl 3-Chloropropionate
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: clear
Phenyl 3-Chloropropionate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Color: Colorless - Pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 157 °C/4kPa
Flash Point: No data available
Explosive limits
No data available
Lower:
Upper: No data available
1.20
Density:
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Phenyl 3-Chloropropionate
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:3-氯丙酸苯酯 在 aluminum (III) chloride 、 lithium aluminium tetrahydride 、 sodium azide 、 potassium carbonate 作用下, 以 四氢呋喃 、 二氯甲烷 、 丙酮 为溶剂, 反应 20.0h, 生成 8-甲氧基-1,2,3,4-四氢异喹啉参考文献:名称:一类酰胺结构的GPR40激动剂化合物及其用途摘要:本发明涉及一类结构新颖的酰胺类化合物及其药物组合物,所述酰胺类化合物的结构如通式(I)所示。该酰胺类化合物(I)能够调节GPR40活性,可用于GPR40活性相关的疾病如糖尿病和代谢综合症。公开号:CN109666027A
-
作为产物:参考文献:名称:Synthesis and evaluation of 7-hydroxyindan-1-one-derived chiral auxiliaries摘要:一系列新型手性缩醛由7-羟基茚满-1-酮和多种取代的手性非外消旋C2-对称1,2-乙二醇(R=甲基、苯基、CH2OMe、CH2OBn、CH2O(1-Np)和异丙基)制备而成。这些缩醛被评估为手性辅助剂,用于不对称合成。在二乙基铝氯化物促进的丙烯酸酯衍生物(R=异丙基)与环戊二烯的Diels-Alder反应中观察到高度的立体化学诱导(91:9 dr)。这表明这些缩醛可以作为有效的不对称底物导向反应中的手性导向剂。关键词:7-羟基茚满-1-酮、手性非外消旋C2-对称1,2-二醇、缩醛、手性辅助剂、Diels-Alder反应。DOI:10.1139/v05-052
-
作为试剂:描述:3-氯丙酰氯 、 苯酚 在 乙酸乙酯 、 sodium hydroxide 、 水 、 Brine 、 Sodium sulfate-III 、 crude compound 、 silica gel 、 ethyl acetate n-hexane 、 3-氯丙酸苯酯 作用下, 以 ice water 为溶剂, 反应 16.0h, 以to obtain the title compound phenyl 3-chloropropanoate (2.5 g, 25% yield) as a colorless liquid的产率得到3-氯丙酸苯酯参考文献:名称:SELECTIVE OCTAHYDRO-CYCLOPENTA[C] PYRROLE NEGATIVE MODULATORS OF NR2B摘要:本发明公开了选择性负向调节含有NR1/NR2B亚基的NMDA受体的化合物,包括该化合物的制药组合物,以及使用该化合物治疗疾病的方法。公开号:US20150225342A1
文献信息
-
Efficient AcrH <sub>2</sub> Catalyzed β‐Trifluoromethylation of Carbonyl Compounds by Atom Transfer Radical Addition Reactions作者:Zi‐Peng Rao、Yu‐Yang Sun、Xin‐Feng Zhou、Qiang Xie、Hui‐Xia Zhu、Jian‐Jun Dai、Jun Xu、Hua‐Jian XuDOI:10.1002/cjoc.201900221日期:2019.10The use of organic catalysis AcrH2 enables the direct β‐trifluoromethylation of carbonyl compounds by atom transfer radical addition reactions with broad substrate scopes under mild conditions. This operationally simple and robust protocol successfully converts a variety of β‐chloro carbonyl compounds into the corresponding α‐chloro‐β‐fluoroalkylcarbonyl products. A significant advantage of this method
-
Synthesis and antibacterial activity of 3-benzylamide derivatives as FtsZ inhibitors作者:Zhongping Hu、Shasha Zhang、Weicheng Zhou、Xiang Ma、Guangya XiangDOI:10.1016/j.bmcl.2017.02.032日期:2017.4series of PC190723 derivatives was synthesized and investigated for their antimicrobial activity. The compounds exhibited good activity against several Gram-positive bacteria as determined by comparison of diameters of the zone of inhibition of test compounds and standard antibiotics. Compound 9 with a fluorine substitution on the phenyl ring showed the best antibacterial activity in the series against
-
[EN] INDANE AND INDOLINE DERIVATIVES AND THE USE THEREOF AS SOLUBLE GUANYLATE CYCLASE ACTIVATORS<br/>[FR] DÉRIVÉS D'INDANE ET D'INDOLINE ET LEUR UTILISATION EN TANT QU'ACTIVATEURS DE LA GUANYLATE CYCLASE SOLUBLE申请人:NOVARTIS AG公开号:WO2016001875A1公开(公告)日:2016-01-07The present invention provides a compound of formula (I) or a pharmaceutically acceptable salt thereof; a method for manufacturing the compounds of the invention, and its therapeutic uses. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.本发明提供了化合物(I)或其药学上可接受的盐;制造本发明化合物的方法及其治疗用途。本发明还提供了药理活性剂的组合和制药组合物。
-
Rhodium(I)-Catalyzed Arylation of β-Chloro Ketones and Related Derivatives through Domino Dehydrochlorination/ Conjugate Addition作者:Quanbin Jiang、Tenglong Guo、Qingfu Wang、Ping Wu、Zhengkun YuDOI:10.1002/adsc.201200821日期:2013.6.17Highly efficient arylations of β‐chloro ketones and their ester and amide derivatives were achieved by means of domino dehydrochlorination/Rh(I)‐catalyzed conjugate addition. In situ generated vinyl ketones and their analogues were identified as the reaction intermediates. The present synthetic protocol provides a concise route to (chiral) β‐aryl ketones, esters, and amides.
-
[EN] ION CHANNEL ANTAGONISTS/BLOCKERS AND USES THEREOF<br/>[FR] ANTAGONISTES/BLOQUEURS DES CANAUX IONIQUES ET LEURS UTILISATIONS申请人:SHANGHAI EAST HOSPITAL公开号:WO2021114313A1公开(公告)日:2021-06-17Provided are ion channel antagonists/blockers and uses thereof. Specifically, it provides the compounds of formula (I) or pharmaceutically acceptable salts, stereoisomers, solvates or prodrugs, preparation method therefor and application thereof. Definition of each group in the formula can be found in the specification for details. Provided is also pharmaceutical composition useful for treatment of heart disease and other ion channel related diseases.提供了离子通道拮抗剂/阻断剂及其用途。具体而言,提供了式(I)的化合物或药用盐、立体异构体、溶剂合物或前药,其制备方法及应用。每个式中的各个基团的定义可在说明书中找到详细信息。还提供了用于治疗心脏病和其他离子通道相关疾病的药物组合物。
表征谱图
-
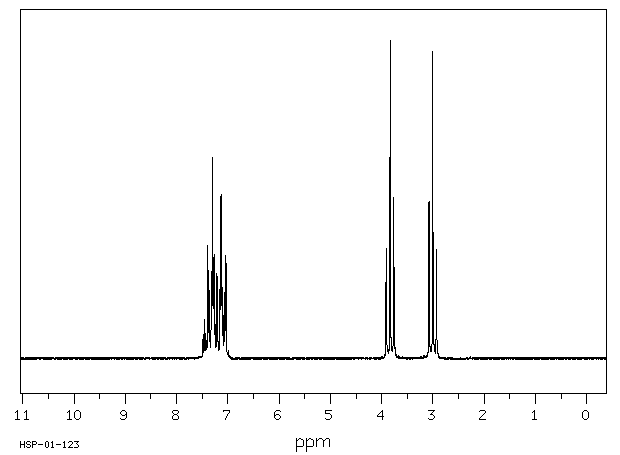
氢谱1HNMR
-
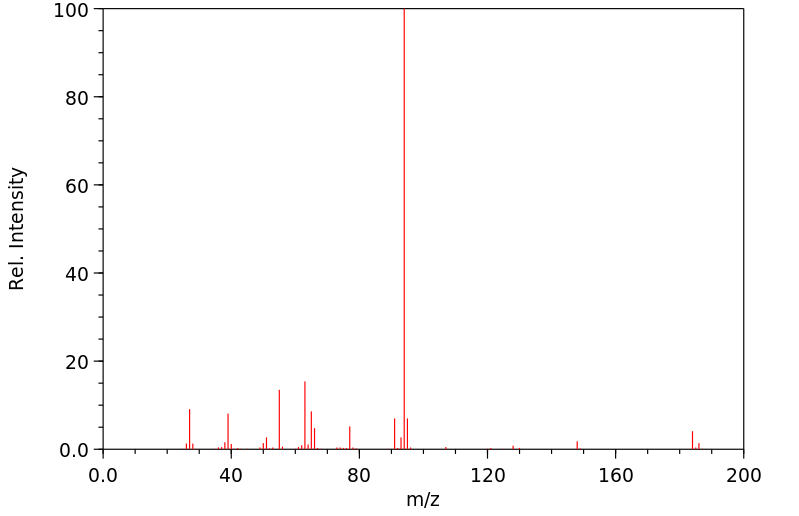
质谱MS
-
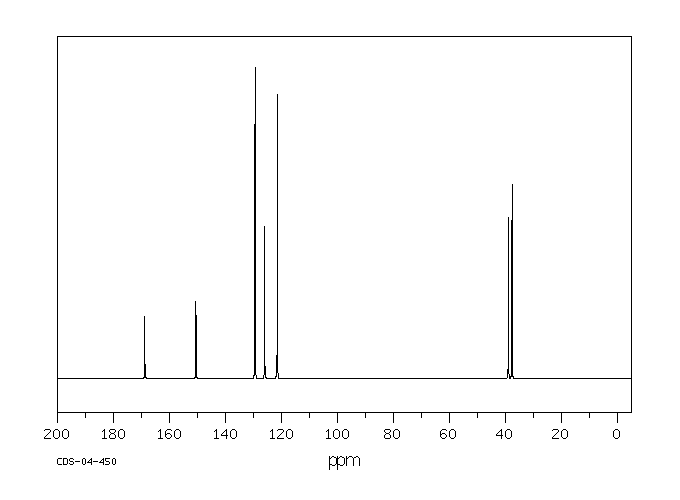
碳谱13CNMR
-
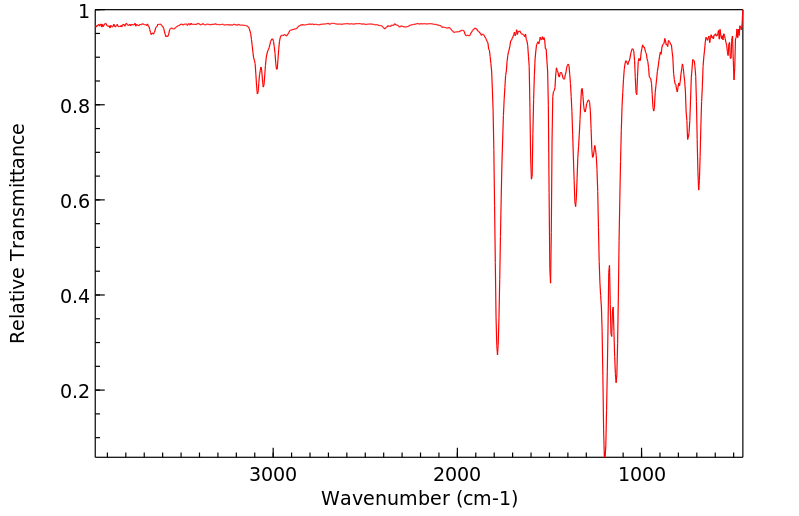
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)