3-苯甲酰基吡啶 | 5424-19-1
中文名称
3-苯甲酰基吡啶
中文别名
苯基-3-吡啶基甲酮;3-苯甲酰吡啶
英文名称
3-Benzoylpyridine
英文别名
phenyl(pyridin-3-yl)methanone
CAS
5424-19-1
化学式
C12H9NO
mdl
MFCD00006394
分子量
183.21
InChiKey
RYMBAPVTUHZCNF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:36-40 °C(lit.)
-
沸点:307 °C(lit.)
-
密度:1.1261 (rough estimate)
-
闪点:302 °F
-
溶解度:溶于甲醇
-
LogP:1.880
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:30
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:25kgs
-
RTECS号:OB6401000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:常温、避光、存放在阴凉干燥处并密封保存。
SDS
| Name: | 3-Benzoylpyridine 97% Material Safety Data Sheet |
| Synonym: | Phenyl-3-pyridyl ketone; Ketone, phenyl 3-pyridyl; Methanone, phenyl-3-pyridinyl-; Pyridine, 3-benzoyl-; 3-Pyridyl phenyl keton |
| CAS: | 5424-19-1 |
Synonym:Phenyl-3-pyridyl ketone; Ketone, phenyl 3-pyridyl; Methanone, phenyl-3-pyridinyl-; Pyridine, 3-benzoyl-; 3-Pyridyl phenyl keton
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5424-19-1 | 3-Benzoylpyridine | 97 | 226-561-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5424-19-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Chunks
Color: white to pale yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 307 deg C @ 760.00mmHg
Freezing/Melting Point: 36.00 - 40.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: 150 deg C ( 302.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C12H9NO
Molecular Weight: 183.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, chloride fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5424-19-1: OB6401000 LD50/LC50:
Not available.
Carcinogenicity:
3-Benzoylpyridine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 5424-19-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5424-19-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5424-19-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (2-溴苯基)-吡啶-3-基甲酮 2-bromophenyl 3-pyridyl ketone 77744-06-0 C12H8BrNO 262.106 3-苄基吡啶 3-benzylpyridine 620-95-1 C12H11N 169.226 —— β-Benzoylisonicotinic acid 74975-28-3 C13H9NO3 227.219 苯基吡啶-3-基甲醇 1-phenyl-1-(pyrid-3-yl)methanol 6270-47-9 C12H11NO 185.225 3-(1-苯乙烯基)吡啶 3-(1-phenylvinyl)pyridine 74309-58-3 C13H11N 181.237 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-benzoylpyridine N-oxide 29022-36-4 C12H9NO2 199.209 3-(3-溴苯甲酰基)吡啶 3-bromophenyl 3-pyridyl ketone 79362-44-0 C12H8BrNO 262.106 3-氨基苯基-3'-吡啶基甲酮 3-(3-aminobenzoyl)pyridine 79568-06-2 C12H10N2O 198.224 3-苯甲酰基-2-氯吡啶 3-benzoyl-2-chloro-pyridine 80099-81-6 C12H8ClNO 217.655 3-苄基吡啶 3-benzylpyridine 620-95-1 C12H11N 169.226 —— 3-nitrophenyl 3-pyridyl ketone 79568-05-1 C12H8N2O3 228.207 —— 5-benzoyl-2-phenylpyridine 30091-51-1 C18H13NO 259.307 苯基吡啶-3-基甲醇 1-phenyl-1-(pyrid-3-yl)methanol 6270-47-9 C12H11NO 185.225 —— 3-(1-phenylethyl)pyridine 93293-23-3 C13H13N 183.253 —— 3-(1-phenylethyl)pyridine 1375744-40-3 C13H13N 183.253 3-(1-苯乙烯基)吡啶 3-(1-phenylvinyl)pyridine 74309-58-3 C13H11N 181.237 —— 3-(chloro(phenyl)methyl)pyridine 38608-51-4 C12H10ClN 203.671 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:与齐美啶有关的吡啶基烯丙基胺的合成及其对神经元单胺摄取的抑制作用。摘要:抗抑郁药齐美碱[6,(Z)-3-(4-溴苯基)-N,N-二甲基-3-(3-吡啶基)烯丙胺]的类似物是神经元5-羟色胺再摄取的选择性抑制剂,其合成方法如下:为了获得具有顺式构型(相对于吡啶基和烯丙胺)的化合物的几种途径。两种方法利用适当取代的苯甲酰基吡啶作为起始原料。在另外两个途径中,将6中的溴直接置换(CN)或通过相应的硫代衍生物转化为H,Cl,I,Me,SiMe3和SMe。通过UV,1H NMR和镧系元素引起的1H NMR位移确定构型。通过测量小鼠脑切片(体外和体内)中的[3H]去甲肾上腺素和5-羟基[14C]色胺的积累,将这些化合物评估为摄取抑制剂。在顺式系列中,对位取代有利于5-羟基色胺活性,而邻位取代有利于NA活性。对5-羟色胺的体外作用对对位取代基的变化不敏感,而仅用Cl,Br(6)和I观察到明显的体内作用。DOI:10.1021/jm00144a025
-
作为产物:参考文献:名称:Ohba, Setsuya; Sakamoto, Takao; Yamanaka, Hiroshi, Heterocycles, 1990, vol. 31, # 7, p. 1301 - 1308摘要:DOI:
-
作为试剂:参考文献:名称:J. Med. Chem. 1985, 28, 287-294摘要:DOI:
文献信息
-
General Method for the Suzuki–Miyaura Cross-Coupling of Primary Amide-Derived Electrophiles Enabled by [Pd(NHC)(cin)Cl] at Room Temperature作者:Peng Lei、Guangrong Meng、Yun Ling、Jie An、Steven P. Nolan、Michal SzostakDOI:10.1021/acs.orglett.7b03191日期:2017.12.15temperature Suzuki–Miyaura cross-coupling of commonly encountered primary benzamides is reported. A combination of site-selective N,N-di-Boc-activation (tert-butoxycarbonyl activation) of the amide nitrogen with practical air- and moisture-stable, well-defined, and highly reactive [Pd(NHC)(cin)Cl] (NHC = N-heterocyclic carbene; cin = cinnamyl) provides a highly effective route to biaryl ketones from primary据报道,室温下,通常遇到的主要苯甲酰胺的Suzuki-Miyaura交叉偶联是一种通用的,高度选择性的方法。酰胺氮的位点选择性N,N -di-Boc活化(叔丁氧基羰基活化)与稳定的空气和水分稳定的,定义明确的和高反应性的[Pd(NHC)(cin)Cl ](NHC = N-杂环卡宾; cin =肉桂基)提供了一种从伯酰胺高收率制备联芳基酮的高效途径。酰胺酰基交叉偶联首次获得了> 1000的TON。
-
Highly enantioselective carbonyl reduction with borane catalyzed by chiral spiroborate esters derived from chiral beta-aminoalcohols申请人:Ortiz-Marciales Margarita公开号:US20080200672A1公开(公告)日:2008-08-21Novel spiroborate esters derived from non-recemic 1,2-amino alcohols were examined as chiral catalyst in the borane reduction of acetophenone and other aromatic ketones at room temperature. The optically active alcohols were obtained in excellent chemical yields and up to 99% ee with less than 10% catalyst.
-
Heterocyclic substituted isoxazolidines and their use as fungicides申请人:Dow Agrosciences LLC公开号:US06313147B1公开(公告)日:2001-11-06Compounds with fungicidal properties having formula X is CH or nitrogen; R is (C1-C12)alkyl, halo(C1-C12)alkyl, (C2-C8)alkenyl, halo(C2-C8)alkenyl, (C2-C8)alkynyl, halo(C2-C8)alkynyl, (C1-C12)alkoxy(C1-C12)alkyl, (C3-C7)cycloalkyl, halo(C3-C7)cycloalkyl, (C3-C7)cycloalkyl(C1-C4)alkyl, aralkyl, aryloxy(C1-C4)alkyl or heterocyclic; R1 is aryl, heterocyclic or C(R6R7R8). R2 and R3 are each selected from hydrogen, (C1-C12)alkyl, halo(C1-C12)alkyl, (C1-C12)alkoxy, halo(C1-C12)alkoxy, (C3-C7)cycloalkyl, (C3-C7)cycloalkyl(C1-C4)alkyl, aryl, aralkyl, heterocyclic; cyano, and (C1-C4)alkoxycarbonyl; R4 and R5 are each selected from hydrogen, (C1-C12)alkyl, halo(C1-C12)alkyl, (C2-C8)alkenyl, halo(C2-C8)alkenyl, (C2-C8)alkynyl, halo(C2-C8)alkynyl, (C3-C7)cycloalkyl, halo(C3-C7)cycloalkyl, (C3-C7)cycloalkyl(C1-C4)alkyl, aryl, aryloxy(C1-C4)alkyl, aralkyl, heterocyclic, cyano, and (C1-C4)alkoxycarbonyl such that R4 and R5 are not both hydrogen; R6, R7, and R8 are each selected from hydrogen, (C1-C12)alkyl, (C2-C8)alkenyl, (C2-C8)alkynyl, (C1-C12)alkoxy(C1-C12)alkyl, (C3-C7)cycloalkyl, (C3-C7)cycloalkyl(C1-C4)alkyl, aryl, aralkyl, and heterocyclic(C1-C4)alkyl.具有杀真菌性质的化合物的化学式为X是CH或氮;R是(C1-C12)烷基,卤代(C1-C12)烷基,(C2-C8)烯基,卤代(C2-C8)烯基,(C2-C8)炔基,卤代(C2-C8)炔基,(C1-C12)烷氧基(C1-C12)烷基,(C3-C7)环烷基,卤代(C3-C7)环烷基,(C3-C7)环烷基(C1-C4)烷基,芳基烷基,芳基氧基(C1-C4)烷基或杂环;R1是芳基,杂环或C(R6R7R8)。R2和R3分别选自氢,(C1-C12)烷基,卤代(C1-C12)烷基,(C1-C12)烷氧基,卤代(C1-C12)烷氧基,(C3-C7)环烷基,(C3-C7)环烷基(C1-C4)烷基,芳基,芳基烷基,杂环;氰基和(C1-C4)烷氧羰基;R4和R5分别选自氢,(C1-C12)烷基,卤代(C1-C12)烷基,(C2-C8)烯基,卤代(C2-C8)烯基,(C2-C8)炔基,卤代(C2-C8)炔基,(C3-C7)环烷基,卤代(C3-C7)环烷基,(C3-C7)环烷基(C1-C4)烷基,芳基,芳基氧基(C1-C4)烷基,芳基烷基,杂环,氰基和(C1-C4)烷氧羰基,使得R4和R5不同时为氢;R6、R7和R8分别选自氢,(C1-C12)烷基,(C2-C8)烯基,(C2-C8)炔基,(C1-C12)烷氧基(C1-C12)烷基,(C3-C7)环烷基,(C3-C7)环烷基(C1-C4)烷基,芳基,芳基烷基和杂环(C1-C4)烷基。
-
Palladium-Catalyzed Synthesis of Diaryl Ketones from Aldehydes and (Hetero)Aryl Halides via C–H Bond Activation作者:Takayuki Wakaki、Takaya Togo、Daisuke Yoshidome、Yoichiro Kuninobu、Motomu KanaiDOI:10.1021/acscatal.8b00440日期:2018.4.6We developed a palladium-catalyzed C–H transformation that enabled the synthesis of ketones from aldehydes and (hetero)aryl halides. The use of picolinamide ligands was key to achieving the transformation. Heteroaryl ketones, as well as diaryl ketones, were synthesized in good to excellent yields, even in gram-scale, using this reaction. Results of density functional theory (DFT) calculations support
-
FLUOROALKYLATING AGENT申请人:IHARA CHEMICAL INDUSTRY CO., LTD.公开号:US20170197920A1公开(公告)日:2017-07-13Problem to be Solved It is intended to provide an industrially preferable fluoroalkylating agent and use thereof. Solution The present invention provides a fluoroalkylating agent represented by the general formula (1) wherein R 1 is a C1 to C8 fluoroalkyl group; R 2 and R 3 are each independently a C1 to C12 alkyl group or the like; Y 1 to Y 4 are each independently a hydrogen atom, a halogen atom, or the like; and X − is a monovalent anion. A compound of the general formula (3): R 4 —S—R 1 having an introduced C1 to C8 fluoroalkyl group is easily obtained by reacting a compound of the general formula (2): R 4 —S—Z wherein R 4 is a hydrocarbon group or the like; and Z is a leaving group, with the compound of the general formula (1).要解决的问题 旨在提供一种工业上可取的氟烷基化剂及其使用方法。 解决方案 本发明提供了一种由通式(1)表示的氟烷基化剂,其中R 1 是C1到C8的氟烷基团;R 2 和R 3 分别独立地是C1到C12的烷基团或类似物;Y 1 到Y 4 分别独立地是氢原子、卤素原子或类似物;X − 是一价阴离子。 通式(3)的化合物:R 4 —S—R 1 ,其中引入了C1到C8的氟烷基团,可通过将通式(2)的化合物:R 4 —S—Z(其中R 4 是烃基团或类似物;Z是离去基团)与通式(1)的化合物反应而轻松获得。
表征谱图
-
氢谱1HNMR
-
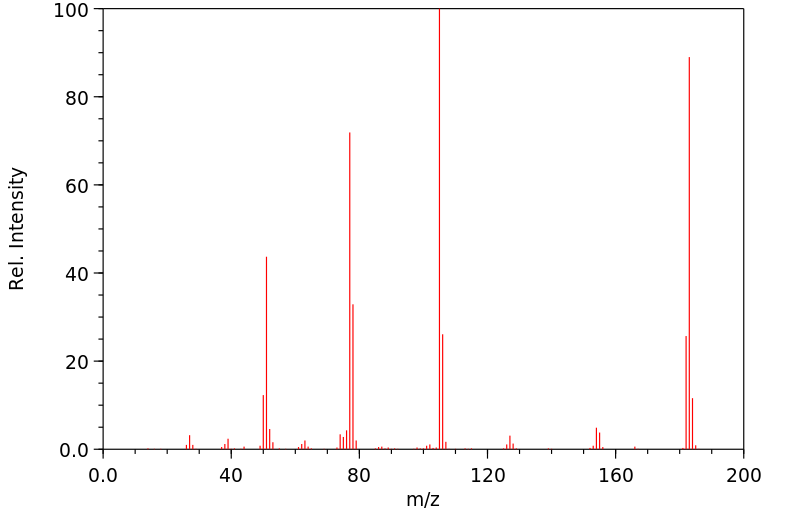
质谱MS
-
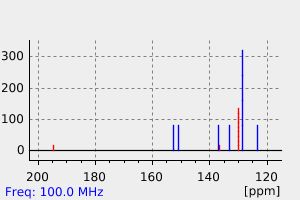
碳谱13CNMR
-
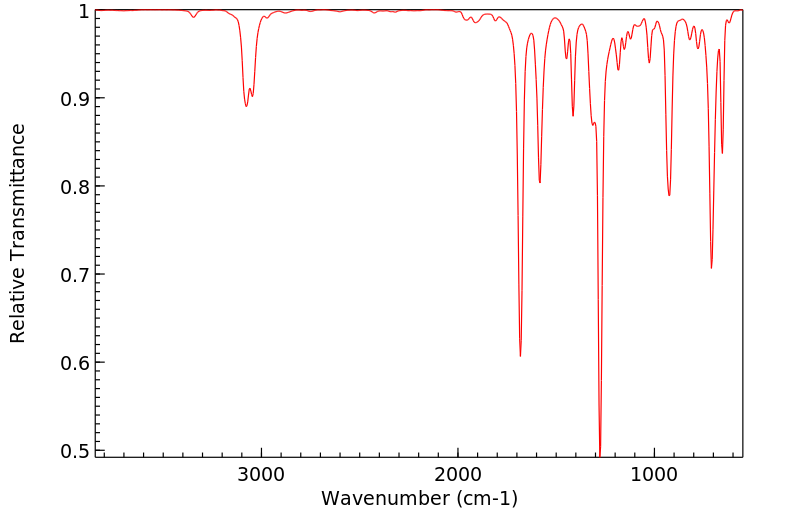
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷