4-丙氧基苯二甲腈 | 106144-18-7
中文名称
4-丙氧基苯二甲腈
中文别名
1,2-二氰基-4-丙氧基苯;4-丙氧基邻苯二甲腈;4-正丙氧基邻苯二甲腈;4-正丙氧基苯二甲腈;4-正丙氧基酞腈
英文名称
4-n-propyloxyphthalonitrile
英文别名
4-propoxyphthalonitrile;4-propoxybenzene-1,2-dicarbonitrile
CAS
106144-18-7
化学式
C11H10N2O
mdl
MFCD00059102
分子量
186.213
InChiKey
RDLZRCSOLOTEPK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:69-70 °C
-
沸点:366.2±32.0 °C(Predicted)
-
密度:1.12±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.272
-
拓扑面积:56.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2926909090
SDS
4-丙氧基苯二甲腈 修改号码:5
模块 1. 化学品
产品名称: 4-Propoxyphthalonitrile
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
4-丙氧基苯二甲腈 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-丙氧基苯二甲腈
百分比: >95.0%(GC)
CAS编码: 106144-18-7
俗名: 1,2-Dicyano-4-propoxybenzene
分子式: C11H10N2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
4-丙氧基苯二甲腈 修改号码:5
模块 7. 操作处置与储存
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 热甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
4-丙氧基苯二甲腈 修改号码:5
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4-Propoxyphthalonitrile
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
4-丙氧基苯二甲腈 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-丙氧基苯二甲腈
百分比: >95.0%(GC)
CAS编码: 106144-18-7
俗名: 1,2-Dicyano-4-propoxybenzene
分子式: C11H10N2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
4-丙氧基苯二甲腈 修改号码:5
模块 7. 操作处置与储存
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 热甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
4-丙氧基苯二甲腈 修改号码:5
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Novel Synthesis of Phthalocyanines from Phthalonitriles under Mild Conditions摘要:通过在 100 °C 的 DMF 中使用金属盐和六甲基二硅氮烷 (HMDS) 进行处理,可以方便地从邻苯二腈中制备酞菁。该反应提供了一种在温和条件下制备多种金属酞菁和取代酞菁以及锌萘酞菁的新方法。DOI:10.1055/s-2002-34237
-
作为产物:描述:参考文献:名称:氟化和非氟化丙醇取代的酞菁锌的光物理和光化学性质摘要:描述了从 4,5-二氯邻苯二甲腈、4-硝基邻苯二甲腈和 3-硝基邻苯二甲腈取代 2,2,3,3-四氟-1-丙醇和正丙醇获得的对称氟化和非氟化锌 (II) 酞菁衍生物的合成。首次报道了氟化和非氟化取代锌(II)酞菁的光物理化学性质的比较。新化合物已通过元素分析、IR、1H NMR 和 19F NMR 光谱、电子光谱和质谱进行表征。在二甲基亚砜 (DMSO) 中研究了化合物的光物理和光化学性质。配合物用苯醌 (BQ) 猝灭,并在相同的溶剂中研究了它们的荧光猝灭性能。还报道了取代数量和位置对锌(II)酞菁 1a-7a 的光物理和光化学参数的影响。酞菁配合物的光物理和光化学特性对于癌症应用的光动力疗法 (PDT) 非常有用。特别是,高单线态氧量子产率对于 II 型机制非常重要。这些配合物具有良好的单线态氧量子产率,并显示出作为 II 型光敏剂的潜力。DOI:10.1002/ejic.200901096
文献信息
-
Preparation of Bis(phthalocyaninato)lutetium with Various Substituents and Their Electrochemical Properties作者:Yunqi Liu、Kiyotaka Shigehara、Akira YamadaDOI:10.1246/bcsj.65.250日期:1992.1A series of bis(phthalocyaninato)lutetium complexes with substituents on each benzoring [Lu(pc2R8)] was prepared and the electrochromic behavior investigated by means of cyclic voltammetry of coated electrodes.Although the unsubstituted complex, Lu(pc)2, showed multistep electrochromism, its stability was quite unsatisfactory due to a falling-off of films from the electrode surface upon repetitive potential scanning. In the case of R = t-butyl, the improved solubility in organic solvents brought about an easier filming process and better adhesion to the electrode surface, though its high oxidation potential caused a cleavage of bi-peripheral complex. In contrast, complexes with electron-donating groups, such as R = propoxy or neopentyloxy, showed a lower oxidation potential and better stability upon repetitive potential scans, though the oxidized color was brownish rather than bright red. Complexes with 4 groups each of tetra-t-butyl and tetra propoxy had the oxidation potential close to that of octapropoxy or octakis(neopentyloxy) complexes and showed much improved stability and electrochromic behavior.制备了一系列苯并环上带有取代基的双(酞菁)镥配合物[Lu(pc2R8)],并通过涂层电极的循环伏安法研究了其电致变色行为。尽管未取代的配合物Lu(pc)2表现出多步电致变色现象,由于重复电位扫描时薄膜从电极表面脱落,其稳定性非常不令人满意。在R=叔丁基的情况下,在有机溶剂中溶解度的提高带来了更容易的成膜过程和对电极表面更好的附着力,尽管其高氧化电位导致双外围络合物的裂解。相比之下,具有给电子基团(例如R =丙氧基或新戊氧基)的配合物在重复电位扫描时表现出较低的氧化电位和更好的稳定性,尽管氧化后的颜色为棕色而不是亮红色。四叔丁基和四丙氧基各有 4 个基团的配合物具有接近八丙氧基或八(新戊氧基)配合物的氧化电位,并且表现出大大改善的稳定性和电致变色行为。
-
Design, synthesis, and biological activity of diiminoisoindolines as complement component 3a antagonists作者:Eugene B. Grant、Deodialsingh Guiadeen、Monica Singer、Dennis Argentieri、Dennis J. Hlasta、Michael WachterDOI:10.1016/s0960-894x(01)00522-4日期:2001.11The failure to fully regulate the inflammation response has been linked to diseases such as rheumatoid arthritis, septic shock syndrome, and asthma. The human complement system initiates and regulates the inflammation response through a cascade of regulatory factors, Complement Component 3a (C3a) is an essential regulatory factor and inhibiting its binding to a C3a receptor will diminish the inflammation response by disrupting the cascade. We report the design, synthesis, in vitro and in vivo activity of diiminoisoindolines as C3a antagonists. (C) 2001 Elsevier Science Ltd. All rights reserved.
-
Electrochemistry and electrochromic behavior of Langmuir-Blodgett films of octakis-substituted rare-earth metal diphthalocyanines作者:Yunqi Liu、Kiyotaka Shigehara、Masahiko Hara、Akira YamadaDOI:10.1021/ja00002a009日期:1991.1Heavy rare-earth metal diphthalocyanine complexes with alkoxy or alkyl substituents dissolved in common organic solvents formed stable Langmuir-Blodgett (L-B) films. The cyclic voltammogram of L-B films on indium-tin oxide Nesa glass electrodes showed smaller peak splitting and more symmetrical shape than those of corresponding solvent-cast films. The multicolor electrochromism was found both in L-B film and cast film systems in contact with an aqueous HCl-KCl electrolyte. For the completion of the color change, different potentials should be applied with different substituents. More than 1 V (versus SCE) was necessary for the tert-butyl-substituted complexes and less than 0.8 V for the propoxy-substituted ones. After about 7 h of repetitive cycling at 100 mV/s between 0 and 0.6 approximately 0.9 V, the relative Q-band intensity decreased by 2-5%, demonstrating good electrochemical stability.
-
US7470797B2申请人:——公开号:US7470797B2公开(公告)日:2008-12-30
-
US7517904B2申请人:——公开号:US7517904B2公开(公告)日:2009-04-14
表征谱图
-
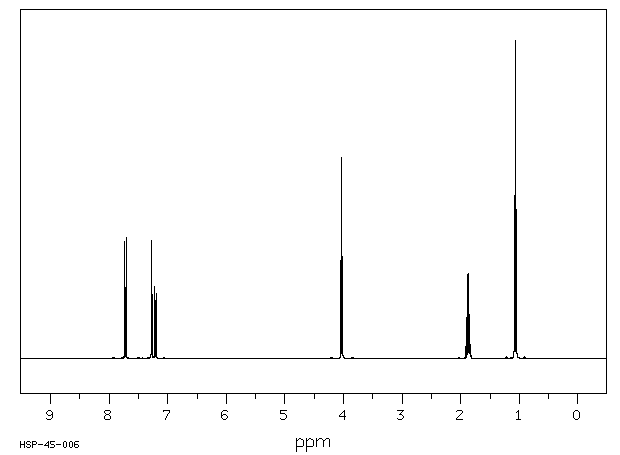
氢谱1HNMR
-
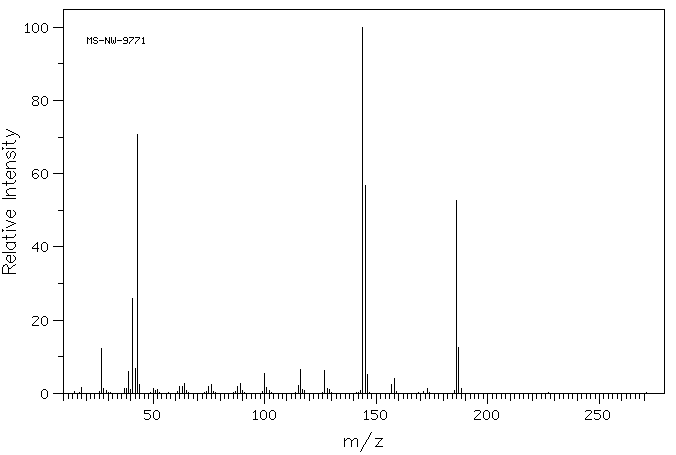
质谱MS
-
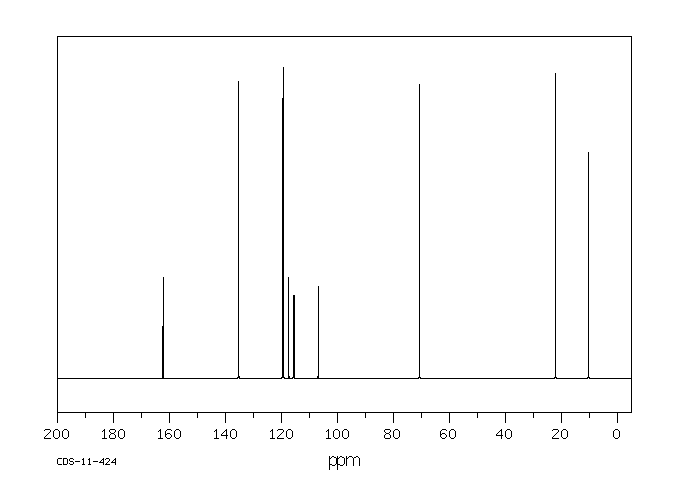
碳谱13CNMR
-
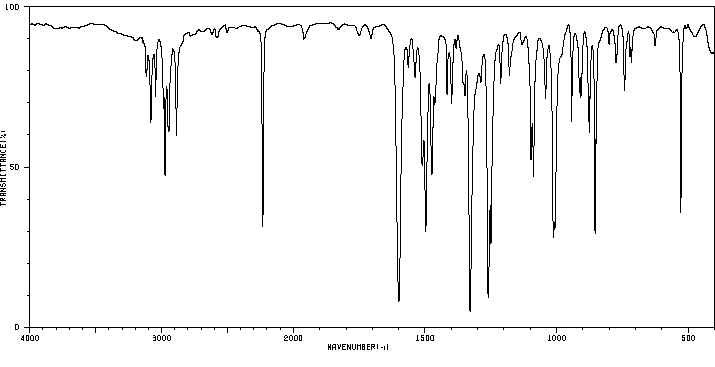
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯