4-乙基-1,3-二恶烷-2-酮 | 4437-85-8
中文名称
4-乙基-1,3-二恶烷-2-酮
中文别名
4-乙基-1,3-二噁烷-2-酮;碳酸1,2-丁烯酯
英文名称
1,2-butylene carbonate
英文别名
4-ethyl-1,3-dioxolan-2-one;butylene carbonate;butene carbonate;1,2-butene carbonate
CAS
4437-85-8
化学式
C5H8O3
mdl
——
分子量
116.117
InChiKey
ZZXUZKXVROWEIF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-45 °C
-
沸点:251 °C
-
密度:1.141
-
闪点:135 °C
-
LogP:0.099 (est)
-
稳定性/保质期:
避免与不相容材料接触,尤其是强氧化剂。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:3.2
-
安全说明:S24/25
-
包装等级:III
-
危险类别:3.2
-
危险品运输编号:UN 1993
-
危险性防范说明:P264,P280,P302+P352,P337+P313,P305+P351+P338,P362+P364,P332+P313
-
危险性描述:H315,H319
SDS
1,2-Butylene Carbonate Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 1,2-Butylene Carbonate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1,2-Butylene Carbonate
Percent: >98.0%(GC)
CAS Number: 4437-85-8
Synonyms: 4-Ethyl-1,3-dioxolan-2-one
Chemical Formula: C5H8O3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
1,2-Butylene Carbonate
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: clear
Color: Colorless - Very pale yellow
Odor: Characteristic
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 74 °C/0.2kPa
Flash Point: 135°C
Explosive limits
Lower: No data available
Upper: No data available
Vapor Density: 4
Density: 1.15
Solubility: No data available
Autoignition Temperature: 421°C
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
1,2-Butylene Carbonate
Section 10. STABILITY AND REACTIVITY
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: skn-rbt 500 mg MLD
eye-rbt 100 mg MLD
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: JI4582000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
No data available
log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
1,2-Butylene Carbonate
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 1,2-Butylene Carbonate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1,2-Butylene Carbonate
Percent: >98.0%(GC)
CAS Number: 4437-85-8
Synonyms: 4-Ethyl-1,3-dioxolan-2-one
Chemical Formula: C5H8O3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
1,2-Butylene Carbonate
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: clear
Color: Colorless - Very pale yellow
Odor: Characteristic
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 74 °C/0.2kPa
Flash Point: 135°C
Explosive limits
Lower: No data available
Upper: No data available
Vapor Density: 4
Density: 1.15
Solubility: No data available
Autoignition Temperature: 421°C
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
1,2-Butylene Carbonate
Section 10. STABILITY AND REACTIVITY
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: skn-rbt 500 mg MLD
eye-rbt 100 mg MLD
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: JI4582000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
No data available
log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
1,2-Butylene Carbonate
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:4-乙基-1,3-二恶烷-2-酮 在 potassium tert-butylate 、 氢气 、 C16H18BrCoINO2 作用下, 以 二丁醚 为溶剂, 160.0 ℃ 、6.0 MPa 条件下, 反应 20.0h, 以92%的产率得到1,2-丁二醇参考文献:名称:明确定义的Cp * Co(III)催化的碳酸盐和聚碳酸酯加氢摘要:我们在此报告了使用定义明确,对空气稳定的高价钴络合物将碳酸酯和聚碳酸酯催化氢化为相应的二醇/醇。首次分离了几种带有N,O螯合的新型Cp * Co(III)配合物,并通过包括单晶X射线晶体学在内的各种光谱技术对其结构进行了表征。这些新颖的Co(III)配合物表现出优异的催化活性,以产生增值的二醇/使用分子氢作为唯一还原剂或从碳酸盐醇和聚碳酸酯通过氢化我PrOH作为转移加氢源。为了证明所开发的方法的实际适用性,我们在既定的反应条件下,使用无磷,富含地球,空气和水分稳定的高价钴催化剂,通过加氢回收了光盘(CD)中的双酚A单体。DOI:10.1002/cctc.202001490
-
作为产物:描述:参考文献:名称:Process for preparing alkylene carbonates摘要:揭示了一种制备烷基碳酸酯的过程,包括将相应的烷基二醇和尿素反应,可选地在含有锡化合物的催化剂存在下进行,反应方程式如下:##STR1##其中R代表含有1至16个碳原子的烷基基团。公开号:US05003084A1
-
作为试剂:描述:参考文献:名称:锌(II)卟啉基离子多孔有机聚合物(iPOP)具有丰富的双功能位点,可促进CO2与环氧化物的环加成摘要:通过吡啶功能化自由基共聚合成了一系列锌(II)卟啉基离子多孔有机聚合物[ZnTPyPBr 4 /DVB(1:m)-iPOP (m = 10, 20, 30, 40 ) ]通过溶剂热法成功表征了锌(II)卟啉(ZnTPyPBr 4 )和二乙烯基苯(DVB)。iPOPs具有高比表面积、较高的CO 2吸附能力、丰富的双功能位点(Zn和Br - )以及在温和条件(80 ℃,0.5 MPa CO 2 )下对CO 2与环氧化物环加成反应的高催化效率。 )。特别地,ZnTPyPBr 4/DVB(1:30)-iPOP对CO 2和环氧氯丙烷的环加成反应表现出最高的催化活性,转化率为99% ,对不同环氧化物具有广泛的适用性,并且可以循环使用5次而几乎不损失活性。值得注意的是,动力学实验表明,在没有溶剂和助催化剂的情况下, ZnTPyPBr 4 /DVB(1:30)-iPOP对于CO 2和环氧氯丙烷的环加成反应表现出较低的活化能(22DOI:10.1016/j.apcata.2023.119380
文献信息
-
Bifunctional One-Component Catalysts for the Addition of Carbon Dioxide to Epoxides作者:Hendrik Büttner、Kornelia Lau、Anke Spannenberg、Thomas WernerDOI:10.1002/cctc.201402816日期:2015.2Several bifunctional ammonium salts were synthesized and employed as one‐component catalysts for the conversion of CO2 and epoxides to produce cyclic carbonates. These catalysts show superior activities compared to their monofunctional analogs. A turnover number of up to 693 and a turnover frequency of up 392 h−1 could be achieved for the best catalyst. Moreover, the effect of various solvents has
-
Microwave-Assisted Synthesis of Tris-Anderson Polyoxometalates for Facile CO<sub>2</sub> Cycloaddition作者:Wei-Dong Yu、Yin Zhang、Yu-Yang Han、Bin Li、Sai Shao、Le-Ping Zhang、Hong-Ke Xie、Jun YanDOI:10.1021/acs.inorgchem.1c00019日期:2021.3.15(NH4)4[ZnMo6O18(C4H8NO3)(OH)3]·4H2O (1), (NH4)4[CuMo6O18(C4H8NO3)(OH)3]·4H2O (2), (TBA)3(NH4)[ZnMo6O17(C5H9O3)2(OH)]·10H2O (3) (TBA = n-C16H36N), and (NH4)4[CuMo6O18(C5H9O3)2]·16H2O (4), were synthesized by a microwave-assisted method. Single-crystal X-ray diffraction revealed that 1 and 2 contained a tris (trihydroxyl organic compounds) ligand grafted on one side, while two tris ligands were grafted on two sides to四个新的tris-Anderson多金属氧酸盐(POMs)(NH 4)4 [ZnMo 6 O 18(C 4 H 8 NO 3)(OH)3 ]·4H 2 O(1),(NH 4)4 [CuMo 6 O 18(C 4 H 8 NO 3)(OH)3 ]·4H 2 O(2),(TBA)3(NH 4)[ZnMo 6 O 17(C 5 H 9 O 3)2(OH)]·10H 2 O(3)(TBA = n -C 16 H 36 N)和(NH 4)4 [CuMo 6 O 18(C 5 H 9 O 3)2 ]·16H 2 O (4)是通过微波辅助法合成的。X射线单晶衍射表明,1和2的一侧接有一个tris(三羟基有机化合物)配体,而两侧的两个tris配体接枝了,在3和3中形成χ/δ和δ/δ异构体。分别为4。首次获得χ/δ异构体3的1 H和13 C NMR光谱,其中6个亚甲基在1 H NMR光谱中显示6个峰,在13
-
Hydrogenation of CO<sub>2</sub> -Derived Carbonates and Polycarbonates to Methanol and Diols by Metal-Ligand Cooperative Manganese Catalysis作者:Viktoriia Zubar、Yury Lebedev、Luis Miguel Azofra、Luigi Cavallo、Osama El-Sepelgy、Magnus RuepingDOI:10.1002/anie.201805630日期:2018.10.8The first base‐metal‐catalysed hydrogenation of CO2‐derived carbonates to alcohols is presented. The reaction proceeds under mild conditions in the presence of a well‐defined manganese complex with a loading as low as 0.25 mol %. The non‐precious‐metal homogenous catalytic system provides an indirect route for the conversion of CO2 into methanol with the co‐production of value‐added (vicinal) diols
-
Bifunctional Ionic Liquids for the Multitask Fixation of Carbon Dioxide into Valuable Chemicals作者:Vitthal B. Saptal、Bhalchandra M. BhanageDOI:10.1002/cctc.201501044日期:2016.1(i) cycloaddition reactions of CO2/CS2 with epoxides to form cyclic carbonates and 1,3‐oxathiolane‐2‐thiones, (ii) transesterification of cyclic carbonates with methanol to form dimethyl carbonate, and (iii) synthesis of quinazoline‐2,4(1 H,3 H)‐diones and quinazoline‐2,4(1 H,3 H)‐dithiones from 2‐aminobenzonitriles and CO2/CS2. The developed methodology is transition‐metal free, solvent free, and additive free. Remarkably合成了一系列特定任务的离子液体(IL),例如单离子,双离子和聚合物支持的离子液体。这些IL用作多任务有机催化剂,可通过一系列反应将二氧化碳转化为有价值的化学物质,包括(i)CO 2 / CS 2与环氧化物的环加成反应,形成环状碳酸酯和1,3-氧杂硫杂环戊烷-2-硫酮, (ii)与甲醇的环状碳酸酯的酯交换反应,以形成碳酸二甲酯,和(iii)喹唑啉-2,4(1合成 ħ,3 ħ) -二酮和喹唑啉-2,4( ħ,3 ħ)从-dithiones 2-氨基苄腈和CO 2 / CS 2。开发的方法是无过渡金属,无溶剂和无添加剂的。值得注意的是,已开发的白介素可在多达七个连续的循环中进行回收。因此,使该协议绿色环保并具有成本效益。
-
Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide by Using Bifunctional One-Component Phosphorus-Based Organocatalysts作者:Hendrik Büttner、Johannes Steinbauer、Thomas WernerDOI:10.1002/cssc.201500612日期:2015.8.24Numerous bifunctional organocatalysts were synthesized and tested for the atom‐efficient addition of carbon dioxide and epoxides to produce cyclic carbonates. These catalysts are based on phosphonium salts containing an alcohol moiety in the side chain for substrate activation through hydrogen bonding. In the model reaction, converting 1,2‐butylene oxide with CO2, 19 catalysts were tested to determine
表征谱图
-
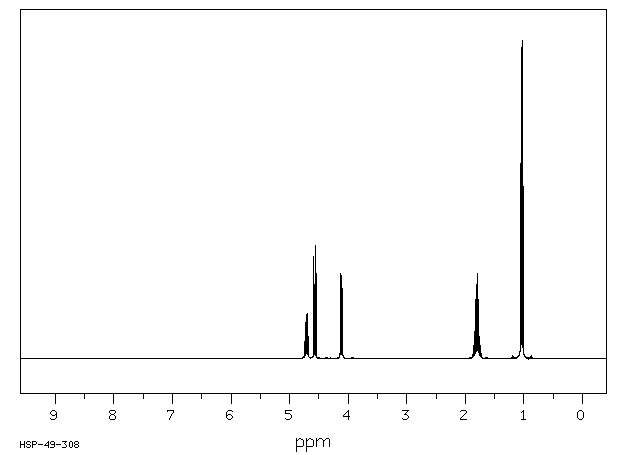
氢谱1HNMR
-
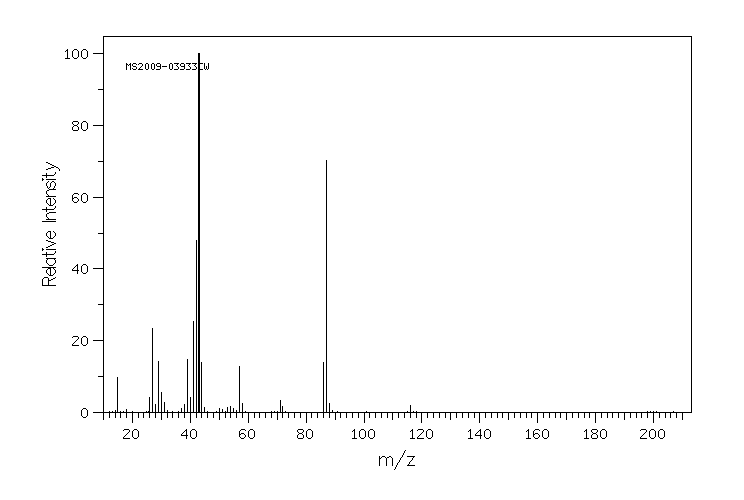
质谱MS
-
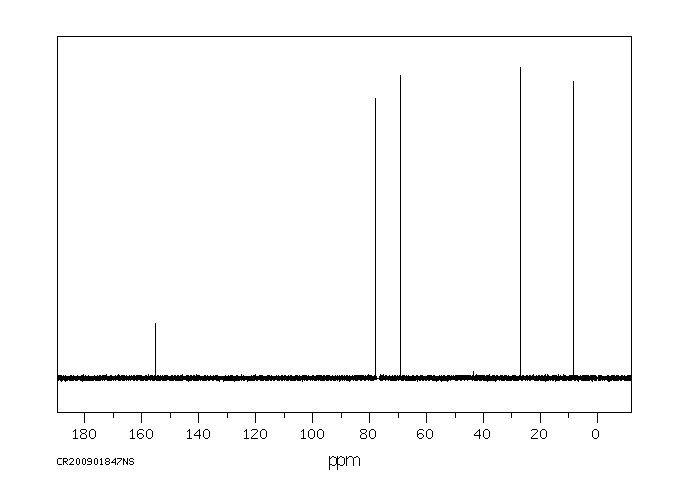
碳谱13CNMR
-
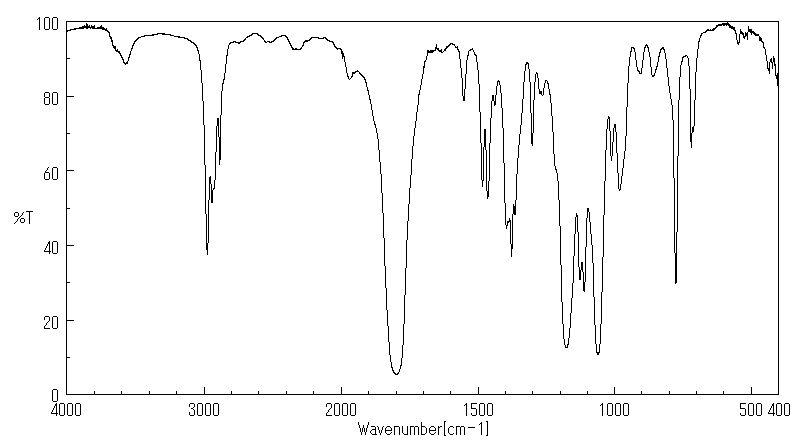
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-4-[(甲基氨基甲酰)氨基]环己烷羧酸
顺式-3-己烯醇碳酸甲酯
镏碳酸盐二水
镍,[碳酸(2-)-κO]-
镁(1-甲基-3-氧代-丁-1-烯基)碳酸氢酯
锌氮烷碳酸盐
锆碳酸盐氧化物
锂(1-羧基环丙基)锂
铵铜碳酸盐
铯碳酸氢钠
铝镁加
铝镁加
铝碳酸镁
铝碳酸镁
钠脲氯酸盐
钠甲基碳酸酯
钙钠碳酸氢盐氟化物
钙四镁钠碳酸氢盐三碳酸盐四氢氧化物
钐(+3)阳离子碳酸酯
重质碳酸镁
重碳酸钠-13C
酸氧(-2)阴离子铅杂亚酸碳
酮羧酸
邻苯二甲酸氢壬酯
过氧碳酸钠
过氧碳酸二钠盐
过氧碳酸,O,O'-1,6-亚己基-OO,OO'-二叔丁基酯
过氧化脲素
过氧化二碳酸双十四酯
过氧化二碳酸双十六酯
过氧化二碳酸二硬脂酰酯
过氧化二碳酸二环己酯
过氧化二碳酸二正丁酯
过氧化二碳酸二异丙酯
过氧化二碳酸二仲丁酯
过氧化二碳酸二乙酯
过氧化二碳酸二-3-甲氧基丁酯
过氧化二碳酸二(2-乙基己)酯
过氧化(2-乙基己基)碳酸叔戊酯
过氧二碳酸二十三烷酯
过氧二碳酸二丙基酯
达比加群酯杂质41
达比加群酯杂质22
达比加群杂质36
达比加群杂质19
辛酰脲
辛基辛氧基甲基碳酸酯
辛基脲
轻质碳酸镁
起始原料2杂质B